Stereoselective synthesis of [2.2]triphenylenophanes via intramolecular double [2 + 2 + 2] cycloadditions
- PMID: 37063805
- PMCID: PMC10094470
- DOI: 10.1039/d3sc00571b
Stereoselective synthesis of [2.2]triphenylenophanes via intramolecular double [2 + 2 + 2] cycloadditions
Abstract
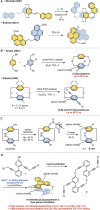
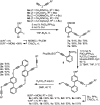
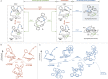
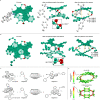
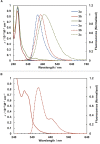
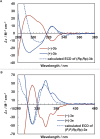
Planar chiral [2.2]cyclophanes with two aromatic rings in close proximity have attracted much attention for their applications as chiral materials and catalysts because of their stable chirality and transannular interactions. Although numerous [2.2]cyclophanes have been synthesized to date, only a few polycyclic aromatic hydrocarbon (PAH)-based ones have been reported, and the simultaneous control of two planar chiralities of the two aromatic rings facing each other has not been achieved. Here we report the enantio- and/or diastereoselective synthesis of planar chiral PAH-based [2.2]cyclophanes ([2.2]triphenylenophanes) via the high-yielding base-mediated intermolecular macrocyclization and Rh- or Ni-catalyzed intramolecular double [2 + 2 + 2] cycloadditions. DFT calculations have revealed that the second [2 + 2 + 2] cycloaddition kinetically determines the diastereoselectivity. Single crystal X-ray diffraction analyses have confirmed that the facing triphenylene or [5]helicene skeletons strongly repel each other, resulting in curved structures with bulged centers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Enantioselective Synthesis of Polycyclic Aromatic Hydrocarbon (PAH)-Based Planar Chiral Bent Cyclophanes by Rhodium-Catalyzed [2+2+2] Cycloaddition.Chemistry. 2020 Oct 1;26(55):12579-12588. doi: 10.1002/chem.202001450. Epub 2020 Sep 17. Chemistry. 2020. PMID: 32350943
-
Enantio-, atrop-, and diastereoselective macrolactonization to access type III cyclophanes.Nat Commun. 2025 Apr 3;16(1):3170. doi: 10.1038/s41467-025-58241-3. Nat Commun. 2025. PMID: 40180958 Free PMC article.
-
Enantio- and Diastereoselective Synthesis and Spiral-Stair-Like Single Helix Assembly of Figure-Eight Cyclophenylenes.Angew Chem Int Ed Engl. 2025 May 26;64(22):e202502764. doi: 10.1002/anie.202502764. Epub 2025 Apr 7. Angew Chem Int Ed Engl. 2025. PMID: 40104859 Free PMC article.
-
Recent theoretical and experimental advances in the electronic circular dichroisms of planar chiral cyclophanes.Top Curr Chem. 2011;298:99-128. doi: 10.1007/128_2010_59. Top Curr Chem. 2011. PMID: 21321800 Review.
-
Recent advances in enantioselective [2 + 2 + 2] cycloaddition.Org Biomol Chem. 2008 Apr 21;6(8):1317-23. doi: 10.1039/b720031e. Epub 2008 Mar 12. Org Biomol Chem. 2008. PMID: 18385836 Review.
Cited by
-
Ir/Zn-cocatalyzed chemo- and atroposelective [2+2+2] cycloaddition for construction of C─N axially chiral indoles and pyrroles.Sci Adv. 2023 Dec 22;9(51):eadk1704. doi: 10.1126/sciadv.adk1704. Epub 2023 Dec 20. Sci Adv. 2023. PMID: 38117883 Free PMC article.
-
Isothiourea-Catalysed Acylative Kinetic and Dynamic Kinetic Resolution of Planar Chiral Paracyclophanols.Angew Chem Int Ed Engl. 2025 Jul 28;64(31):e202507126. doi: 10.1002/anie.202507126. Epub 2025 Jun 23. Angew Chem Int Ed Engl. 2025. PMID: 40392605 Free PMC article.
References
-
-
For reviews on cyclophanes, see:
- Roy I. David A. H. G. Das P. J. Pe D. J. Stoddart J. F. Chem. Soc. Rev. 2022;51:5557–5605. doi: 10.1039/D0CS00352B. - DOI - PubMed
- López R. Palomo C. Angew. Chem., Int. Ed. 2022;61:e202113504. - PMC - PubMed
- Wu J.-R. Yang Y.-W. Chem. Commun. 2019;55:1533–1543. doi: 10.1039/C8CC09374A. - DOI - PubMed
- Kotha S. Shirbhate M. E. Waghule G. T. Beilstein J. Org. Chem. 2015;11:1274–1331. doi: 10.3762/bjoc.11.142. - DOI - PMC - PubMed
- Ramaiah D. Neelakandan P. P. Nair A. K. Avirah R. R. Chem. Soc. Rev. 2010;39:4158–4168. doi: 10.1039/B920032K. - DOI - PubMed
-
-
- Cram D. J. Allinger N. L. Steinberg H. J. Am. Chem. Soc. 1954;76:6132–6141. doi: 10.1021/ja01652a081. - DOI
-
-
For reviews on [2.2]paracyclophanes, see:
- Teng J.-M. Zhang D.-W. Chen C.-F. ChemPhotoChem. 2022;6:e202100228. doi: 10.1002/cptc.202100228. - DOI
- Felder S. Wu S. Brom J. Micouin L. Benedetti E. Chirality. 2021;33:506–527. doi: 10.1002/chir.23335. - DOI - PubMed
- Hassan Z. Spuling E. Knoll D. M. Bräse S. Angew. Chem., Int. Ed. 2020;59:2156–2170. doi: 10.1002/anie.201904863. - DOI - PMC - PubMed
- Morisaki Y. Chujo Y. Bull. Chem. Soc. Jpn. 2019;92:265–274. doi: 10.1246/bcsj.20180259. - DOI
- Hassan Z. Spuling E. Knoll D. M. Lahann J. Bräse S. Chem. Soc. Rev. 2018;47:6947–6963. doi: 10.1039/C7CS00803A. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources

