Pre-clinical models to define correlates of protection for SARS-CoV-2
- PMID: 37063834
- PMCID: PMC10097995
- DOI: 10.3389/fimmu.2023.1166664
Pre-clinical models to define correlates of protection for SARS-CoV-2
Abstract
A defined immune profile that predicts protection against a pathogen-of-interest, is referred to as a correlate of protection (CoP). A validated SARS-CoV-2 CoP has yet to be defined, however considerable insights have been provided by pre-clinical vaccine and animal rechallenge studies which have fewer associated limitations than equivalent studies in human vaccinees or convalescents, respectively. This literature review focuses on the advantages of the use of animal models for the definition of CoPs, with particular attention on their application in the search for SARS-CoV-2 CoPs. We address the conditions and interventions required for the identification and validation of a CoP, which are often only made possible with the use of appropriate in vivo models.
Keywords: SARS-CoV-2; animal models; correlates of protection (CoP); immunity; vaccines.
Copyright © 2023 Brady, Tipton, Longet and Carroll.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


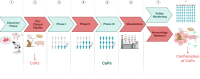
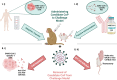

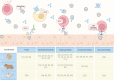
References
-
- WHO director-general's opening remarks at the media briefing on COVID-19 - 11 march 2020 (2020). Available at: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- WHO . COVID-19 dashboard (2020). Available at: https://covid19.who.int/.
-
- The European Agency for the Evaluation of Medicinal Products CfPMP . Guideline on influenza vaccines non-clinical and clinical module (EMA/CHMP/VWP/457259/2014) (2016). Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/influenza-va....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

