Comparable cellular and humoral immunity upon homologous and heterologous COVID-19 vaccination regimens in kidney transplant recipients
- PMID: 37063863
- PMCID: PMC10102365
- DOI: 10.3389/fimmu.2023.1172477
Comparable cellular and humoral immunity upon homologous and heterologous COVID-19 vaccination regimens in kidney transplant recipients
Abstract
Background: Kidney transplant recipients (KTRs) are at high risk for a severe course of coronavirus disease 2019 (COVID-19); thus, effective vaccination is critical. However, the achievement of protective immunogenicity is hampered by immunosuppressive therapies. We assessed cellular and humoral immunity and breakthrough infection rates in KTRs vaccinated with homologous and heterologous COVID-19 vaccination regimens.
Method: We performed a comparative in-depth analysis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T-cell responses using multiplex Fluorospot assays and SARS-CoV-2-specific neutralizing antibodies (NAbs) between three-times homologously (n = 18) and heterologously (n = 8) vaccinated KTRs.
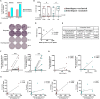
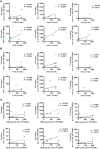
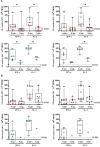
Results: We detected SARS-CoV-2-reactive T cells in 100% of KTRs upon third vaccination, with comparable frequencies, T-cell expression profiles, and relative interferon γ and interleukin 2 production per single cell between homologously and heterologously vaccinated KTRs. SARS-CoV-2-specific NAb positivity rates were significantly higher in heterologously (87.5%) compared to homologously vaccinated (50.0%) KTRs (P < 0.0001), whereas the magnitudes of NAb titers were comparable between both subcohorts after third vaccination. SARS-CoV-2 breakthrough infections occurred in equal numbers in homologously (38.9%) and heterologously (37.5%) vaccinated KTRs with mild-to-moderate courses of COVID-19.
Conclusion: Our data support a more comprehensive assessment of not only humoral but also cellular SARS-CoV-2-specific immunity in KTRs to provide an in-depth understanding about the COVID-19 vaccine-induced immune response in a transplant setting.
Keywords: COVID-19 vaccination; T-cell responses; immunosuppressive therapy; kidney transplant recipients; multiplex Fluorospot; neutralizing antibodies.
Copyright © 2023 Körber, Holzmann-Littig, Wilkens, Liao, Werz, Platen, Cheng, Tellenbach, Kappler, Lehner, Mijočević, Christa, Assfalg, Heemann, Schmaderer, Protzer, Braunisch, Bauer and Renders.
Conflict of interest statement
UP received personal fees from Abbott, Abbvie, Arbutus, Gilead, GSK, J&J, MSD, Roche, Sanofi, Sobi, and Vaccitech. UP is a cofounder and share-holder of SCG Cell Therapy. The lab of UP received grants from Hoehnle AG and SCG Cell Therapy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

