C-type lectin receptor agonists elicit functional IL21-expressing Tfh cells and induce primary B cell responses in neonates
- PMID: 37063899
- PMCID: PMC10102809
- DOI: 10.3389/fimmu.2023.1155200
C-type lectin receptor agonists elicit functional IL21-expressing Tfh cells and induce primary B cell responses in neonates
Abstract
Introduction: C-type lectin receptor (CLR) agonists emerged as superior inducers of primary B cell responses in early life compared with Toll-like receptor (TLR) agonists, while both types of adjuvants are potent in adults.
Methods: Here, we explored the mechanisms accounting for the differences in neonatal adjuvanticity between a CLR-based (CAF®01) and a TLR4-based (GLA-SE) adjuvant administered with influenza hemagglutinin (HA) in neonatal mice, by using transcriptomics and systems biology analyses.
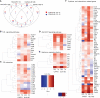
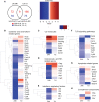
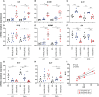
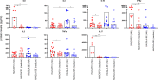
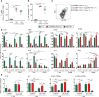
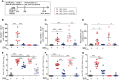
Results: On day 7 after immunization, HA/CAF01 increased IL6 and IL21 levels in the draining lymph nodes, while HA/GLA-SE increased IL10. CAF01 induced mixed Th1/Th17 neonatal responses while T cell responses induced by GLA-SE had a more pronounced Th2-profile. Only CAF01 induced T follicular helper (Tfh) cells expressing high levels of IL21 similar to levels induced in adult mice, which is essential for germinal center (GC) formation. Accordingly, only CAF01- induced neonatal Tfh cells activated adoptively transferred hen egg lysozyme (HEL)-specific B cells to form HEL+ GC B cells in neonatal mice upon vaccination with HEL-OVA.
Discussion: Collectively, the data show that CLR-based adjuvants are promising neonatal and infant adjuvants due to their ability to harness Tfh responses in early life.
Keywords: C-type lectin receptor agonists; T follicular helper (Tfh); interleukin-21 (IL-21); interleukin-6 (IL-6); neonatal vaccinology; toll-like receptor agonists; vaccine adjuvants.
Copyright © 2023 Vono, Mastelic-Gavillet, Mohr, Östensson, Persson, Olafsdottir, Lemeille, Pejoski, Hartley, Christensen, Andersen, Didierlaurent, Harandi, Lambert and Siegrist.
Conflict of interest statement
DC and PA are co-inventors on patent applications covering CAF01. As employees, DC and PA have assigned all rights to Statens Serum Institut, a Danish non-profit governmental institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Pihlgren M, Tougne C, Bozzotti P, Fulurija A, Duchosal MA, Lambert PH, et al. . Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J Immunol (2003) 170:2824–32. doi: 10.4049/jimmunol.170.6.2824 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

