The role of autophagy in cardiovascular disease: Cross-interference of signaling pathways and underlying therapeutic targets
- PMID: 37063954
- PMCID: PMC10090687
- DOI: 10.3389/fcvm.2023.1088575
The role of autophagy in cardiovascular disease: Cross-interference of signaling pathways and underlying therapeutic targets
Abstract
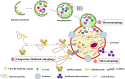
Autophagy is a conserved lysosomal pathway for the degradation of cytoplasmic proteins and organelles, which realizes the metabolic needs of cells and the renewal of organelles. Autophagy-related genes (ATGs) are the main molecular mechanisms controlling autophagy, and their functions can coordinate the whole autophagic process. Autophagy can also play a role in cardiovascular disease through several key signaling pathways, including PI3K/Akt/mTOR, IGF/EGF, AMPK/mTOR, MAPKs, p53, Nrf2/p62, Wnt/β-catenin and NF-κB pathways. In this paper, we reviewed the signaling pathway of cross-interference between autophagy and cardiovascular diseases, and analyzed the development status of novel cardiovascular disease treatment by targeting the core molecular mechanism of autophagy as well as the critical signaling pathway. Induction or inhibition of autophagy through molecular mechanisms and signaling pathways can provide therapeutic benefits for patients. Meanwhile, we hope to provide a unique insight into cardiovascular treatment strategies by understanding the molecular mechanism and signaling pathway of crosstalk between autophagy and cardiovascular diseases.
Keywords: autophagy; autophagy-related gene; cardiovascular disease; crosstalk; potential target; signaling pathway.
© 2023 Jiang, Zhou, Yang, Wang, Feng, Wang, Xu, Jing, Wang, Su, Yang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

