Comparative cardiotoxicity risk of pembrolizumab versus nivolumab in cancer patients undergoing immune checkpoint inhibitor therapy: A meta-analysis
- PMID: 37064101
- PMCID: PMC10090546
- DOI: 10.3389/fonc.2023.1080998
Comparative cardiotoxicity risk of pembrolizumab versus nivolumab in cancer patients undergoing immune checkpoint inhibitor therapy: A meta-analysis
Abstract
Objective: Recently, several researchers have reported the incidence of cardiac-related toxicities occurring with nivolumab (Opdivo) and pembrolizumab (Keytruda). There is still a need for balance between oncology treatment efficacy and reduction of cardiotoxicity burden in immune checkpoint inhibitor (ICI)-treated patients. Thus, the primary aim was to determine whether pembrolizumab or nivolumab would present with a greater risk for cardiotoxicity reports.
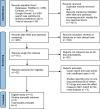
Materials and methods: This meta-analysis was performed with respect to the MOOSE reporting guidelines. Studies were retrieved by searching PubMed, Embase, and Google Scholar; the search terms were Keytruda or Pembrolizumab, PD1 inhibitors, anti-PD1 drugs, Nivolumab or Opdivo, and cardiotoxicities or cardiac toxicity. The study was restricted to original articles investigating ICI-induced cardiac immune-related adverse events (irAEs). The targeted population was cancer patients treated with either pembrolizumab or nivolumab monotherapy, of which those with records of any cardiac events following the therapy were labeled as events. The measures used to achieve the comparison were descriptive proportions, probabilities, and meta-analysis pooled odds ratios (ORs).
Results: Fifteen studies were included in this meta-analysis. Nivolumab accounted for 55.7% cardiotoxicity and pembrolizumab, for 27.31% (P = 0.027). The meta-analysis was based on the Mantel-Haenszel method, and the random-effect model yielded a pooled OR = 0.73 (95% CI [0.43-1.23] P = 0.24), with considerable heterogeneity (I2 = 99% P = 0). Hence, the difference in cardiotoxicity odds risk between pembrolizumab and nivolumab was not statistically significant. On subgroup analysis based on cardiotoxicity type, the "myocarditis" subgroup in which there was no statistical heterogeneity was associated with a significant cardiotoxicity risk increase with pembrolizumab (OR = 1.30 [1.07;1.59], P< 0.05; I2 = 0%, Ph = 0.4).
Conclusion: To our knowledge, this is the first meta-analysis to compare the cardiotoxicity potentials of nivolumab and pembrolizumab. In contrast to previous reports, the overall findings here demonstrated that nivolumab-induced cardiotoxicity was more commonly reported in the literature than pembrolizumab; however, myocarditis seemed more likely to occur with pembrolizumab therapy.
Keywords: cardiotoxicity; meta-analysis; nivolumab; pembrolizumab; risk.
Copyright © 2023 Ndjana Lessomo, Wang and Mukuka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. . Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; (2022). Available at: https://gco.iarc.fr/today (Accessed February 2021).
Publication types
LinkOut - more resources
Full Text Sources