A recombinant adenovirus vector containing the synNotch receptor gene for the treatment of triple-negative breast cancer
- PMID: 37064130
- PMCID: PMC10090503
- DOI: 10.3389/fonc.2023.1147668
A recombinant adenovirus vector containing the synNotch receptor gene for the treatment of triple-negative breast cancer
Abstract
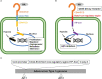
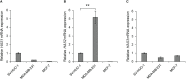
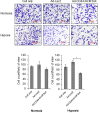
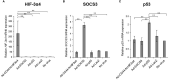
Triple-negative breast cancer (TNBC) is known as the most difficult molecular subtype of breast cancer to treat. Recent studies revealed that cancer stem cells (CSCs) play a critical role in TNBC recurrence and metastasis. In this study, we developed a recombinant replication-deficient adenoviral vector (Ad-CD44-N-HIF-3α4), which contains a gene encoding a synthetic Notch (synNotch) receptor composed of the extracellular domain of CD44 (CD44-ECD) and the hypoxia-inducible factor (HIF)-3α4 connected by the Notch core regulatory region. CD44 is a transmembrane glycoprotein and known as a CSC marker in breast cancer and other malignancies. HIF-3α4 is a dominant-negative regulator of HIF-1α and HIF-2α and inhibits hypoxia-inducing effect. Both CD44 and HIF signals contribute cancer stemness and maintaining CSCs in breast cancer. The CD44-ECD in the synNotch receptor acts as the CD44 decoy receptor, and after a ligand such as a hyaluronic acid binds to the CD44-ECD, HIF-3α4 is released from the Notch core domain. We performed an in vivo study using a mouse xenograft model of MDA-MB-231, a highly invasive TNBC cell, and confirmed the significant antitumor activity of the intratumoral injections of Ad-CD44-N-HIF3α4. Our findings in this study warrant the further development of Ad-CD44-N-HIF3α4 for the treatment of patients with TNBC.
Keywords: HIF; adenovirus; breast cancer; cancer stem cells; gene therapy.
Copyright © 2023 A, Kunimura, Tominaga, Hirata, Nishioka, Uesugi, Yamazaki, Ueki, Kitagawa, Fujisawa and Shirakawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
A conditionally replicative adenovirus vector containing the synNotch receptor gene for the treatment of muscle-invasive bladder cancer.Cancer Gene Ther. 2025 Mar;32(3):306-317. doi: 10.1038/s41417-025-00879-8. Epub 2025 Feb 26. Cancer Gene Ther. 2025. PMID: 40011711 Free PMC article.
-
HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling.World J Stem Cells. 2020 Jan 26;12(1):87-99. doi: 10.4252/wjsc.v12.i1.87. World J Stem Cells. 2020. PMID: 32110277 Free PMC article.
-
Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer.J Mol Med (Berl). 2014 Feb;92(2):151-64. doi: 10.1007/s00109-013-1102-5. Epub 2013 Nov 20. J Mol Med (Berl). 2014. PMID: 24248265 Free PMC article.
-
The Milk Protein Alpha-Casein Suppresses Triple Negative Breast Cancer Stem Cell Activity Via STAT and HIF-1alpha Signalling Pathways in Breast Cancer Cells and Fibroblasts.J Mammary Gland Biol Neoplasia. 2019 Sep;24(3):245-256. doi: 10.1007/s10911-019-09435-1. Epub 2019 Sep 12. J Mammary Gland Biol Neoplasia. 2019. PMID: 31529195
-
Targeting hypoxia-inducible factor-1alpha: A new strategy for triple-negative breast cancer therapy.Biomed Pharmacother. 2022 Dec;156:113861. doi: 10.1016/j.biopha.2022.113861. Epub 2022 Oct 10. Biomed Pharmacother. 2022. PMID: 36228375 Review.
Cited by
-
Ad-VT causes ovarian cancer A2780 cell death via mitochondrial apoptosis and autophagy pathways.Transl Oncol. 2024 Oct;48:102067. doi: 10.1016/j.tranon.2024.102067. Epub 2024 Aug 1. Transl Oncol. 2024. PMID: 39094512 Free PMC article.
-
A conditionally replicative adenovirus vector containing the synNotch receptor gene for the treatment of muscle-invasive bladder cancer.Cancer Gene Ther. 2025 Mar;32(3):306-317. doi: 10.1038/s41417-025-00879-8. Epub 2025 Feb 26. Cancer Gene Ther. 2025. PMID: 40011711 Free PMC article.
-
Targeting FOXP3 Tumor-Intrinsic Effects Using Adenoviral Vectors in Experimental Breast Cancer.Viruses. 2023 Aug 25;15(9):1813. doi: 10.3390/v15091813. Viruses. 2023. PMID: 37766222 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

