Automatic tumor segmentation and metachronous single-organ metastasis prediction of nasopharyngeal carcinoma patients based on multi-sequence magnetic resonance imaging
- PMID: 37064158
- PMCID: PMC10099248
- DOI: 10.3389/fonc.2023.953893
Automatic tumor segmentation and metachronous single-organ metastasis prediction of nasopharyngeal carcinoma patients based on multi-sequence magnetic resonance imaging
Abstract
Background: Distant metastases is the main failure mode of nasopharyngeal carcinoma. However, early prediction of distant metastases in NPC is extremely challenging. Deep learning has made great progress in recent years. Relying on the rich data features of radiomics and the advantages of deep learning in image representation and intelligent learning, this study intends to explore and construct the metachronous single-organ metastases (MSOM) based on multimodal magnetic resonance imaging.
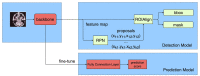
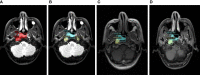
Patients and methods: The magnetic resonance imaging data of 186 patients with nasopharyngeal carcinoma before treatment were collected, and the gross tumor volume (GTV) and metastatic lymph nodes (GTVln) prior to treatment were defined on T1WI, T2WI, and CE-T1WI. After image normalization, the deep learning platform Python (version 3.9.12) was used in Ubuntu 20.04.1 LTS to construct automatic tumor detection and the MSOM prediction model.
Results: There were 85 of 186 patients who had MSOM (including 32 liver metastases, 25 lung metastases, and 28 bone metastases). The median time to MSOM was 13 months after treatment (7-36 months). The patients were randomly assigned to the training set (N = 140) and validation set (N = 46). By comparison, we found that the overall performance of the automatic tumor detection model based on CE-T1WI was the best (6). The performance of automatic detection for primary tumor (GTV) and lymph node gross tumor volume (GTVln) based on the CE-T1WI model was better than that of models based on T1WI and T2WI (AP@0.5 is 59.6 and 55.6). The prediction model based on CE-T1WI for MSOM prediction achieved the best overall performance, and it obtained the largest AUC value (AUC = 0.733) in the validation set. The precision, recall, precision, and AUC of the prediction model based on CE-T1WI are 0.727, 0.533, 0.730, and 0.733 (95% CI 0.557-0.909), respectively. When clinical data were added to the deep learning prediction model, a better performance of the model could be obtained; the AUC of the integrated model based on T2WI, T1WI, and CE-T1WI were 0.719, 0.738, and 0.775, respectively. By comparing the 3-year survival of high-risk and low-risk patients based on the fusion model, we found that the 3-year DMFS of low and high MSOM risk patients were 95% and 11.4%, respectively (p < 0.001).
Conclusion: The intelligent prediction model based on magnetic resonance imaging alone or combined with clinical data achieves excellent performance in automatic tumor detection and MSOM prediction for NPC patients and is worthy of clinical application.
Keywords: automatic learning; intelligent prediction; metachronous single-organ metastases prediction; multimodal magnetic resonance imaging; nasopharyngeal carcinoma.
Copyright © 2023 Huang, Zhu, Yang, Luo, Zhang, Yang, Ren, Ren, Lang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Magnetic resonance imaging based on radiomics for differentiating T1-category nasopharyngeal carcinoma from nasopharyngeal lymphoid hyperplasia: a multicenter study.Jpn J Radiol. 2024 Jul;42(7):709-719. doi: 10.1007/s11604-024-01544-0. Epub 2024 Feb 27. Jpn J Radiol. 2024. PMID: 38409300
-
MRI-based radiomics as response predictor to radiochemotherapy for metastatic cervical lymph node in nasopharyngeal carcinoma.Br J Radiol. 2021 Jun 1;94(1122):20201212. doi: 10.1259/bjr.20201212. Epub 2021 Apr 21. Br J Radiol. 2021. PMID: 33882240 Free PMC article.
-
Development and validation of a prediction model for malignant sinonasal tumors based on MR radiomics and machine learning.Eur Radiol. 2025 Apr;35(4):2074-2083. doi: 10.1007/s00330-024-11033-7. Epub 2024 Aug 30. Eur Radiol. 2025. PMID: 39210161
-
Radiomics Analysis of Multiparametric MRI for Prediction of Synchronous Lung Metastases in Osteosarcoma.Front Oncol. 2022 Feb 22;12:802234. doi: 10.3389/fonc.2022.802234. eCollection 2022. Front Oncol. 2022. PMID: 35273911 Free PMC article.
-
Development and external validation of a multiparametric MRI-based radiomics model for preoperative prediction of microsatellite instability status in rectal cancer: a retrospective multicenter study.Eur Radiol. 2023 Mar;33(3):1835-1843. doi: 10.1007/s00330-022-09160-0. Epub 2022 Oct 25. Eur Radiol. 2023. PMID: 36282309
Cited by
-
Deep Learning for Nasopharyngeal Carcinoma Segmentation in Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis.Bioengineering (Basel). 2024 May 17;11(5):504. doi: 10.3390/bioengineering11050504. Bioengineering (Basel). 2024. PMID: 38790370 Free PMC article. Review.
-
Construction of an oligometastatic prediction model for nasopharyngeal carcinoma patients based on pathomics features and dynamic multi-swarm particle swarm optimization support vector machine.Front Oncol. 2025 Jun 19;15:1589919. doi: 10.3389/fonc.2025.1589919. eCollection 2025. Front Oncol. 2025. PMID: 40612342 Free PMC article.
-
Intravoxel incoherent motion diffusion-weighted imaging in nasopharyngeal carcinoma: comparison of the turbo spin-echo and echo-planar imaging techniques.Quant Imaging Med Surg. 2024 Dec 5;14(12):9101-9111. doi: 10.21037/qims-24-1021. Epub 2024 Nov 29. Quant Imaging Med Surg. 2024. PMID: 39698659 Free PMC article.
-
Radiomics-based lymph nodes prognostic models from three MRI regions in nasopharyngeal carcinoma.Heliyon. 2024 May 18;10(10):e31557. doi: 10.1016/j.heliyon.2024.e31557. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803981 Free PMC article.
-
Deciphering the Prognostic Efficacy of MRI Radiomics in Nasopharyngeal Carcinoma: A Comprehensive Meta-Analysis.Diagnostics (Basel). 2024 Apr 29;14(9):924. doi: 10.3390/diagnostics14090924. Diagnostics (Basel). 2024. PMID: 38732337 Free PMC article.
References
LinkOut - more resources
Full Text Sources

