Dystonia-like behaviors and impaired sensory-motor integration following neurotoxic lesion of the pedunculopontine tegmental nucleus in mice
- PMID: 37064180
- PMCID: PMC10101329
- DOI: 10.3389/fneur.2023.1102837
Dystonia-like behaviors and impaired sensory-motor integration following neurotoxic lesion of the pedunculopontine tegmental nucleus in mice
Abstract
Introduction: The pedunculopontine nucleus (PPTg) is a vital interface between the basal ganglia and cerebellum, participating in modulation of the locomotion and muscle tone. Pathological changes of the PPTg have been reported in patients and animal models of dystonia, while its effect and mechanism on the phenotyping of dystonia is still unknown.
Methods: In this study, a series of behavioral tests focusing on the specific deficits of dystonia were conducted for mice with bilateral and unilateral PPTg excitotoxic lesion, including the dystonia-like movements evaluation, different types of sensory-motor integrations, explorative behaviors and gait. In addition, neural dysfunctions including apoptosis, neuroinflammation, neurodegeneration and neural activation of PPTg-related motor areas in the basal ganglia, reticular formations and cerebellum were also explored.
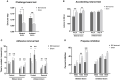
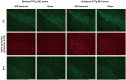
Results: Both bilateral and unilateral lesion of the PPTg elicited dystonia-like behaviors featured by the hyperactivity of the hindlimb flexors. Moreover, proprioceptive and auditory sensory-motor integrations were impaired in bilaterally lesioned mice, while no overt alterations were found for the tactile sensory-motor integration, explorative behaviors and gait. Similar but milder behavioral deficits were found in the unilaterally lesioned mice, with an effective compensation was observed for the auditory sensory-motor integration. Histologically, no neural loss, apoptosis, neuroinflammation and neurodegeneration were found in the substantia nigra pars compacta and caudate putamen (CPu) following PPTg lesion, while reduced neural activity was found in the dorsolateral part of the CPu and striatal indirect pathway-related structures including subthalamic nucleus, globus pallidus internus and substantia nigra pars reticular. Moreover, the neural activity was decreased for the reticular formations such as pontine reticular nucleus, parvicellular reticular nucleus and gigantocellular reticular nucleus, while deep cerebellar nuclei were spared.
Conclusion: In conclusion, lesion of the PPTg could elicit dystonia-like behaviors through its effect on the balance of the striatal pathways and the reticular formations.
Keywords: dystonia; motor performance; pedunculopontine nucleus; reticular formation; sensory-motor integration; striatal pathways.
Copyright © 2023 Su, Hu, Song, Yang, Li, Zhou, Hu, Wan, Teng and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Determination of acetylcholine and dopamine content in thalamus and striatum after excitotoxic lesions of the pedunculopontine tegmental nucleus in rats.Neurosci Lett. 2002 Mar 29;322(1):45-8. doi: 10.1016/s0304-3940(02)00084-8. Neurosci Lett. 2002. PMID: 11958840
-
Direct and indirect nigrofugal projections to the nucleus reticularis pontis caudalis mediate in the motor execution of the acoustic startle reflex.Brain Struct Funct. 2018 Jul;223(6):2733-2751. doi: 10.1007/s00429-018-1654-9. Epub 2018 Mar 24. Brain Struct Funct. 2018. PMID: 29574585
-
The Pedunculopontine Tegmental Nucleus as a Motor and Cognitive Interface between the Cerebellum and Basal Ganglia.Front Neuroanat. 2016 Nov 7;10:109. doi: 10.3389/fnana.2016.00109. eCollection 2016. Front Neuroanat. 2016. PMID: 27872585 Free PMC article. Review.
-
Neurophysiology of the pedunculopontine tegmental nucleus.Neurobiol Dis. 2019 Aug;128:19-30. doi: 10.1016/j.nbd.2018.03.004. Epub 2018 Mar 7. Neurobiol Dis. 2019. PMID: 29524600 Review.
-
Functional disconnection of the substantia nigra pars compacta from the pedunculopontine nucleus impairs learning of a conditioned avoidance task.Neurobiol Learn Mem. 2010 Sep;94(2):229-39. doi: 10.1016/j.nlm.2010.05.011. Epub 2010 Jun 1. Neurobiol Learn Mem. 2010. PMID: 20595069 Free PMC article.
Cited by
-
Novel mouse model of alternating hemiplegia of childhood exhibits prominent motor and seizure phenotypes.Neurobiol Dis. 2024 Dec;203:106751. doi: 10.1016/j.nbd.2024.106751. Epub 2024 Nov 26. Neurobiol Dis. 2024. PMID: 39603281
References
LinkOut - more resources
Full Text Sources
Miscellaneous

