An open-label observational study and meta-analysis of non-invasive vagus nerve stimulation in medically refractory chronic cluster headache
- PMID: 37064192
- PMCID: PMC10098146
- DOI: 10.3389/fneur.2023.1100426
An open-label observational study and meta-analysis of non-invasive vagus nerve stimulation in medically refractory chronic cluster headache
Abstract
Background: Many patients with cluster headache (CH) are inadequately controlled by current treatment options. Non-invasive vagus nerve stimulation (nVNS) is reported to be effective in the management of CH though some studies suggest that it is ineffective.
Objective: To assess the safety and efficacy of nVNS in chronic cluster headache (CCH) patients.
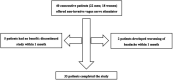
Method: We prospectively analysed data from 40 patients with refractory CCH in this open-label, observational study. Patients were seen in tertiary headache clinics at the National Hospital for Neurology and Neurosurgery and trained to use nVNS as preventative therapy. Patients were reivewed at one month and then three-monthly from onset. The primary endpoint was number of patients achieving ≥50% reduction in attack frequency at 3 months. A meta-analysis of all published studies evaluating the efficacy of nVNS in CCH was also conducted. We searched MEDLINE and EMBASE for all studies investigating the use of nVNS as a preventive or adjunctive treatment for CCH with five or more participants. Combined mean difference and responder proportions with 95% confidence intervals (CI) were calculated from the included studies.
Results: 17/40 patients (43%) achieved ≥50% reduction in attack frequency at 3 months. There was a significant reduction in monthly attack frequency from a baseline of 124 (±67) attacks to 79 (±63) attacks in month 3 (mean difference 44.7; 95% CI 25.1 to 64.3; p < 0.001). In month 3, there was also a 1.2-point reduction in average severity from a baseline Verbal Rating Scale of 8/10 (95% CI 0.5 to 1.9; p = 0.001). Four studies, along with the present study, were deemed eligible for meta-analysis, which showed a responder proportion of 0.35 (95% CI 0.07 to 0.69, n = 137) and a mean reduction in headache frequency of 35.3 attacks per month (95% CI 11.0 to 59.6, n = 108), from a baseline of 105 (±22.7) attacks per month.
Conclusion: This study highlights the potential benefit of nVNS in CCH, with significant reductions in headache frequency and severity. To better characterise the effect, randomised sham-controlled trials are needed to confirm the beneficial response of VNS reported in some, but not all, open-label studies.
Keywords: chronic cluster headache; medically refractory; meta-analysis; non invasive vagus nerve stimulation; vagus nerve (VN) stimulation.
Copyright © 2023 Simmonds, Lagrata, Stubberud, Cheema, Tronvik, Matharu and Kamourieh.
Conflict of interest statement
SL has received payment for attending advisory meetings and development of presentation from Allergan Novartis, Eli Lilly and TEVA. AS is co-founder of Nordic Brain Tech, a company developing a non-pharmacological biofeedback treatment for migraine and holds a pending patent application relating to the company’s product. ET is co-founder and shareholder of Nordic Brain Tech AS and Palion Medical AS. He serves on advisory board for Eli Lilly and Novartis and TEVA. He has received speaker honoraria from Eli Lilly, Novartis, Allergan and TEVA. MM serves on the advisory board for Abbott, Allergan, Eli Lilly, Medtronic, Novartis, TEVA; has received payment for the development of educational presentations from Allergan, electroCore, Eli Lilly, Medtronic, Novartis, and TEVA; and, has received research grants from Abbott, electroCore and Medtronic. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Non-Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache: Findings From the Randomized, Double-Blind, Sham-Controlled ACT1 Study.Headache. 2016 Sep;56(8):1317-32. doi: 10.1111/head.12896. Headache. 2016. PMID: 27593728 Free PMC article. Clinical Trial.
-
Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: A randomized, double-blind, sham-controlled ACT2 study.Cephalalgia. 2018 Apr;38(5):959-969. doi: 10.1177/0333102417744362. Epub 2017 Dec 12. Cephalalgia. 2018. PMID: 29231763 Free PMC article. Clinical Trial.
-
Cervical non-invasive vagus nerve stimulation (nVNS) for preventive and acute treatment of episodic and chronic migraine and migraine-associated sleep disturbance: a prospective observational cohort study.J Headache Pain. 2015;16:101. doi: 10.1186/s10194-015-0582-9. Epub 2015 Dec 3. J Headache Pain. 2015. PMID: 26631234 Free PMC article.
-
Spotlight on cervical vagus nerve stimulation for the treatment of primary headache disorders: a review.J Pain Res. 2018 Aug 27;11:1613-1625. doi: 10.2147/JPR.S129202. eCollection 2018. J Pain Res. 2018. PMID: 30214271 Free PMC article. Review.
-
Review of non-invasive vagus nerve stimulation (gammaCore): efficacy, safety, potential impact on comorbidities, and economic burden for episodic and chronic cluster headache.Am J Manag Care. 2017 Nov;23(17 Suppl):S317-S325. Am J Manag Care. 2017. PMID: 29144717 Review.
Cited by
-
15. Cluster Headache.Pain Pract. 2025 Jun;25(5):e70050. doi: 10.1111/papr.70050. Pain Pract. 2025. PMID: 40437707 Free PMC article. Review.
-
The clinical effect of an implanted vagus nerve stimulator in a patient with chronic cluster headache and focal epilepsy - A case report.Headache. 2025 May 20;65(7):1202-6. doi: 10.1111/head.14963. Online ahead of print. Headache. 2025. PMID: 40391549 Free PMC article.
-
Prospective study of the effect of auricular percutaneous electrical nerve field stimulation on quality of life in children with pain related disorders of gut-brain interaction.Front Pain Res (Lausanne). 2023 Sep 8;4:1223932. doi: 10.3389/fpain.2023.1223932. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37745801 Free PMC article.
-
Vagus nerve stimulation in dementia: A scoping review of clinical and pre-clinical studies.AIMS Neurosci. 2024 Sep 27;11(3):398-420. doi: 10.3934/Neuroscience.2024024. eCollection 2024. AIMS Neurosci. 2024. PMID: 39431268 Free PMC article.
References
-
- Weaver-Agostoni J. Cluster headache. Am Fam Physician. (2013) 88:122–8. - PubMed
LinkOut - more resources
Full Text Sources