External stimulation: A potential therapeutic strategy for tendon-bone healing
- PMID: 37064229
- PMCID: PMC10102526
- DOI: 10.3389/fbioe.2023.1150290
External stimulation: A potential therapeutic strategy for tendon-bone healing
Abstract
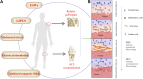
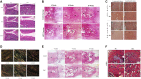
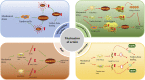
Injuries at the tendon-bone interface are very common in the field of sports medicine, and healing at the tendon-bone interface is complex. Injuries to the tendon-bone interface can seriously affect a patient's quality of life, so it is essential to restore stability and promote healing of the tendon-bone interface. In addition to surgical treatment, the healing of tendons and bones can also be properly combined with extracorporeal stimulation therapy during the recovery process. In this review, we discuss the effects of extracorporeal shock waves (ESWs), low-intensity pulsed ultrasound (LIPUS), and mechanical stress on tendon-bone healing, focusing on the possible mechanisms of action of mechanical stress on tendon-bone healing in terms of transcription factors and biomolecules. The aim is to provide possible therapeutic approaches for subsequent clinical treatment.
Keywords: extracorporeal shock wave; low-intensity pulsed ultrasound; mechanical stress; mechanism of action; tendon-bone healing.
Copyright © 2023 Fu, Lan, Wang, Bao, Qin, Zheng, Liu and Wong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Initiation Timing of Low-Intensity Pulsed Ultrasound Stimulation for Tendon-Bone Healing in a Rabbit Model.Am J Sports Med. 2016 Oct;44(10):2706-2715. doi: 10.1177/0363546516651863. Epub 2016 Jun 29. Am J Sports Med. 2016. PMID: 27358283
-
Acceleration of Bone-Tendon Interface Healing by Low-Intensity Pulsed Ultrasound Is Mediated by Macrophages.Phys Ther. 2021 Jul 1;101(7):pzab055. doi: 10.1093/ptj/pzab055. Phys Ther. 2021. PMID: 33561257
-
Effects of low-intensity pulsed ultrasound on tendon-bone healing in an intra-articular sheep knee model.Arthroscopy. 2007 Feb;23(2):197-204. doi: 10.1016/j.arthro.2006.09.003. Arthroscopy. 2007. PMID: 17276228
-
Augmentation of tendon-to-bone healing.J Bone Joint Surg Am. 2014 Mar 19;96(6):513-21. doi: 10.2106/JBJS.M.00009. J Bone Joint Surg Am. 2014. PMID: 24647509 Review.
-
Effects of Low-Intensity Pulsed Ultrasound in Tendon Injuries.J Ultrasound Med. 2023 Sep;42(9):1923-1939. doi: 10.1002/jum.16230. Epub 2023 Apr 20. J Ultrasound Med. 2023. PMID: 37079603 Review.
Cited by
-
Gradient scaffolds in bone-soft tissue interface engineering: Structural characteristics, fabrication techniques, and emerging trends.J Orthop Translat. 2025 Jan 28;50:333-353. doi: 10.1016/j.jot.2024.10.015. eCollection 2025 Jan. J Orthop Translat. 2025. PMID: 39944791 Free PMC article. Review.
-
Musculoskeletal Biomaterials: Stimulated and Synergized with Low Intensity Pulsed Ultrasound.J Funct Biomater. 2023 Oct 9;14(10):504. doi: 10.3390/jfb14100504. J Funct Biomater. 2023. PMID: 37888169 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

