Development of a biomarker signature using grating-coupled fluorescence plasmonic microarray for diagnosis of MIS-C
- PMID: 37064248
- PMCID: PMC10102909
- DOI: 10.3389/fbioe.2023.1066391
Development of a biomarker signature using grating-coupled fluorescence plasmonic microarray for diagnosis of MIS-C
Abstract
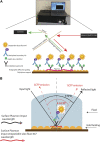
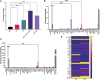
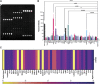
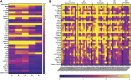
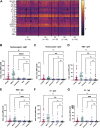
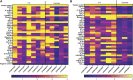
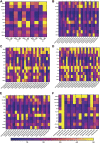
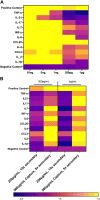
Multisystem inflammatory syndrome in children (MIS-C) is a rare but serious condition that can develop 4-6 weeks after a school age child becomes infected by SARS-CoV-2. To date, in the United States more than 8,862 cases of MIS-C have been identified and 72 deaths have occurred. This syndrome typically affects children between the ages of 5-13; 57% are Hispanic/Latino/Black/non-Hispanic, 61% of patients are males and 100% have either tested positive for SARS-CoV-2 or had direct contact with someone with COVID-19. Unfortunately, diagnosis of MIS-C is difficult, and delayed diagnosis can lead to cardiogenic shock, intensive care admission, and prolonged hospitalization. There is no validated biomarker for the rapid diagnosis of MIS-C. In this study, we used Grating-coupled Fluorescence Plasmonic (GCFP) microarray technology to develop biomarker signatures in pediatric salvia and serum samples from patients with MIS-C in the United States and Colombia. GCFP measures antibody-antigen interactions at individual regions of interest (ROIs) on a gold-coated diffraction grating sensor chip in a sandwich immunoassay to generate a fluorescent signal based on analyte presence within a sample. Using a microarray printer, we designed a first-generation biosensor chip with the capability of capturing 33 different analytes from 80 of sample (saliva or serum). Here, we show potential biomarker signatures in both saliva and serum samples in six patient cohorts. In saliva samples, we noted occasional analyte outliers on the chip within individual samples and were able to compare those samples to 16S RNA microbiome data. These comparisons indicate differences in relative abundance of oral pathogens within those patients. Microsphere Immunoassay (MIA) of immunoglobulin isotypes was also performed on serum samples and revealed MIS-C patients had several COVID antigen-specific immunoglobulins that were significantly higher than other cohorts, thus identifying potential new targets for the second-generation biosensor chip. MIA also identified additional biomarkers for our second-generation chip, verified biomarker signatures generated on the first-generation chip, and aided in second-generation chip optimization. Interestingly, MIS-C samples from the United States had a more diverse and robust signature than the Colombian samples, which was also illustrated in the MIA cytokine data. These observations identify new MIS-C biomarkers and biomarker signatures for each of the cohorts. Ultimately, these tools may represent a potential diagnostic tool for use in the rapid identification of MIS-C.
Keywords: COVID-19; MIS-C; biomarkers; diagnostic; microarray.
Copyright © 2023 Maltz-Matyschsyk, Melchiorre, Herbst, Hogan, Dibble, O’Sullivan, Graf, Jadhav, Lawrence, Lee, Carson, Radolf, Salazar, Lynes and Connecticut Children’s COVID Collaborative.
Conflict of interest statement
ML has intellectual property (U.S. Patent #7,655,421) related to functional phenotyping of leukocytes by SPR microarray. ML, MM-M, DL, and JG have ongoing, NIH-funded, collaborations with Ciencia, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Incidence of Multisystem Inflammatory Syndrome in Children Among US Persons Infected With SARS-CoV-2.JAMA Netw Open. 2021 Jun 1;4(6):e2116420. doi: 10.1001/jamanetworkopen.2021.16420. JAMA Netw Open. 2021. PMID: 34110391 Free PMC article.
-
Detection of faecal SARS-CoV-2 RNA in a prospective cohort of children with multisystem inflammatory syndrome (MIS-C).Epidemiol Prev. 2021;45(6):522-527. doi: 10.19191/EP21.6.120. Epidemiol Prev. 2021. PMID: 34841834 English.
-
Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study.Lancet Child Adolesc Health. 2021 May;5(5):323-331. doi: 10.1016/S2352-4642(21)00050-X. Epub 2021 Mar 10. Lancet Child Adolesc Health. 2021. PMID: 33711293 Free PMC article.
-
SARS-CoV-2 Infection and Racial Disparities in Children: Protective Mechanisms and Severe Complications Related to MIS-C.J Racial Ethn Health Disparities. 2022 Aug;9(4):1536-1542. doi: 10.1007/s40615-021-01092-7. Epub 2021 Jul 13. J Racial Ethn Health Disparities. 2022. PMID: 34255304 Free PMC article. Review.
-
Multisystem Inflammatory Syndrome in Children (MIS-C).Curr Allergy Asthma Rep. 2022 May;22(5):53-60. doi: 10.1007/s11882-022-01031-4. Epub 2022 Mar 22. Curr Allergy Asthma Rep. 2022. PMID: 35314921 Free PMC article. Review.
Cited by
-
Going Viral: Assessing the Impact of Social Media on Enrollment in a Coronavirus Disease 2019 (COVID-19) Cohort Study.Cureus. 2024 Mar 13;16(3):e56096. doi: 10.7759/cureus.56096. eCollection 2024 Mar. Cureus. 2024. PMID: 38618422 Free PMC article.
-
Soil microbiome characterization and its future directions with biosensing.J Biol Eng. 2024 Sep 10;18(1):50. doi: 10.1186/s13036-024-00444-1. J Biol Eng. 2024. PMID: 39256848 Free PMC article.
-
The Detection of COVID-19-Related Multivariate Biomarker Immune Response in Pediatric Patients: Statistical Aspects.Viruses. 2025 Feb 21;17(3):297. doi: 10.3390/v17030297. Viruses. 2025. PMID: 40143228 Free PMC article.
References
-
- Algarni A. S., NjoudAlamri M. N Z. K, R A., Alghamdi S. H., Alsubhi R. S., Alghamdi S. H. (2022). Clinical practice guidelines in multisystem inflammatory syndrome (MIS-C) related to COVID-19: A critical review and recommendations. World J. Pediatr. 18 (2), 83–90. 10.1007/s12519-021-00499-w - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

