Lateral septal nucleus, dorsal part, and dentate gyrus are necessary for spatial and object recognition memory, respectively, in mice
- PMID: 37064302
- PMCID: PMC10102498
- DOI: 10.3389/fnbeh.2023.1139737
Lateral septal nucleus, dorsal part, and dentate gyrus are necessary for spatial and object recognition memory, respectively, in mice
Abstract
Introduction: Cognitive impairment includes the abnormality of learning, memory and judgment, resulting in severe learning and memory impairment and social activity impairment, which greatly affects the life quality of individuals. However, the specific mechanisms underlying cognitive impairment in different behavioral paradigms remain to be elucidated.
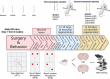
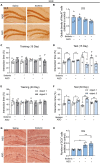
Methods: The study utilized two behavioral paradigms, novel location recognition (NLR) and novel object recognition (NOR), to investigate the brain regions involved in cognitive function. These tests comprised two phases: mice were presented with two identical objects for familiarization during the training phase, and a novel (experiment) or familiar (control) object/location was presented during testing. Immunostaining quantification of c-Fos, an immediate early gene used as a neuronal activity marker, was performed in eight different brain regions after the NLR or NOR test.
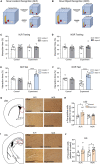
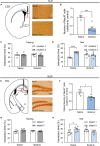
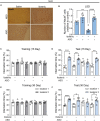
Results: The number of c-Fos-positive cells was significantly higher in the dorsal part of the lateral septal nucleus (LSD) in the NLR and dentate gyrus (DG) in the NOR experiment group than in the control group. We further bilaterally lesioned these regions using excitotoxic ibotenic acid and replenished the damaged areas using an antisense oligonucleotide (ASO) strategy.
Discussion: These data reinforced the importance of LSD and DG in regulating spatial and object recognition memory, respectively. Thus, the study provides insight into the roles of these brain regions and suggests potential intervention targets for impaired spatial and object recognition memory.
Keywords: c-Fos; dentate gyrus; dorsal part; lateral septal nucleus (LSD); lesion; recognition memory.
Copyright © 2023 Jiang, Dong, Dong, Zhu, Pan, Hu, Xu, Xu, Xu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

