Advances in differential diagnosis of cerebrovascular diseases in magnetic resonance imaging: a narrative review
- PMID: 37064346
- PMCID: PMC10102759
- DOI: 10.21037/qims-22-750
Advances in differential diagnosis of cerebrovascular diseases in magnetic resonance imaging: a narrative review
Abstract
Background and objective: Cerebrovascular diseases (CVDs), particularly cerebral stroke, remain a primary cause of disability and death worldwide. Accurate diagnosis of CVDs is essential to guide therapeutic decisions and foresee the prognosis. Different CVDs have different pathological processes while they have many signs in common with some other brain diseases. Thus, differential diagnoses of strokes from other primary and secondary CVDs are especially important and challenging.
Methods: This review is composed mainly based on searching PubMed articles between September, 2013 and December 26, 2022 in English.
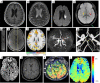
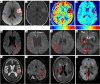
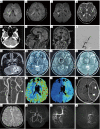
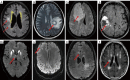
Key content and findings: Neuroimaging is a powerful tool for CVD diagnosis including cerebral angiography, ultrasound, computed tomography, and positron emission tomography as well as magnetic resonance imaging (MRI). MRI excels other imaging techniques by its features of non-invasive, diverse sequences and high spatiotemporal resolution. It can detect hemodynamic, structural alterations of intracranial arteries and metabolic status of their associated brain regions. In acute stroke, differential diagnosis of ischemic from hemorrhagic stroke and other intracranial vasculopathies is a common application of MRI. By providing information about the pathological characteristics of cerebral diseases exhibiting different degrees of behavioral alterations, cognitive impairment, motor dysfunction and other indications, MRI can differentiate strokes from other primary CVDs involving cerebral small vessels and identify vascular dementia from hyponatremia, brain tumors and other secondary or non-primary CVDs.
Conclusions: Recent advances in MRI technology allow clinical neuroimaging to provide unique reference for differentiating many previously inconclusive CVDs. MRI technology is worthy of full exploration while breaking its limitations in clinical applications should be considered.
Keywords: Cerebrovascular diseases (CVDs); cerebral stroke; differential diagnosis; magnetic resonance imaging (MRI); neuroimaging.
2023 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-750/coif). The authors have no conflicts of interest to declare.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
