Review of Mavacamten for Obstructive Hypertrophic Cardiomyopathy and Future Directions
- PMID: 37064432
- PMCID: PMC10094472
- DOI: 10.2147/DDDT.S368590
Review of Mavacamten for Obstructive Hypertrophic Cardiomyopathy and Future Directions
Abstract
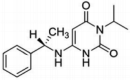
Hypertrophic cardiomyopathy (HCM) is a condition with abnormal hypertrophy of the left ventricle in the absence of common causes. The most common form involves the basal septum and can lead to obstruction of the left ventricular outflow tract. Patients can experience exertional symptoms such as chest pain, dyspnea and syncope. Traditional treatment has included beta blockers and nondihydropyridine calcium channel blockers with second-line therapy being disopyramide. Recently, mavacamten, a cardiac myosin inhibitor, has demonstrated improvement in quantitative measures of obstruction and symptom relief to such a degree that patients were able to defer invasive management of the disease. This review focuses on the pharmacology of mavacamten, its clinical trial data and guidance on how to incorporate this drug into clinical practice. Furthermore, it discusses emerging therapies currently being investigated for HCM.
Keywords: EXPLORER HCM; VALOR HCM; hypertrophic cardiomyopathy; mavacamten; septal reduction therapy.
© 2023 Dong et al.
Conflict of interest statement
Tiffany Dong and Ben Alencherry are co-first authors for this study. Dr Milind Y Desai is a consultant for Bristol Myers Squibb and Medtronic. The authors report no other conflicts of interest in this work.
Figures
References
-
- Administration FD. Approved drug proucts: CAMZYOS (mavacamten) capsules for oral use; 2022.
-
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. Circulation. 2020;142(25):e558–e631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources