Inhibition of miR-20a by pterostilbene facilitates prostate cancer cells killed by NK cells via up-regulation of NKG2D ligands and TGF-β1down-regulation
- PMID: 37064475
- PMCID: PMC10102449
- DOI: 10.1016/j.heliyon.2023.e14957
Inhibition of miR-20a by pterostilbene facilitates prostate cancer cells killed by NK cells via up-regulation of NKG2D ligands and TGF-β1down-regulation
Abstract
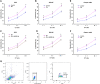
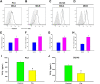
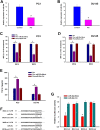
Natural killer (NK) cells play a potent role in antitumor immunity via spontaneously eliminating tumor directly. However, some tumors such as prostate cancer constantly escape this immune response by down-regulating cell surface molecule recognition and/or secreting immune impressive cytokines. Here, we found pterostilbene, a natural agent with potent anticancer activity, could enhance expression of major histocompatibility complex class I chain-related proteins A and B (MICA/B) on prostate cancer cells surface, which are ligands of the natural killer group 2 member D (NKG2D) expressed by NK cells, and inhibit TGF-β1 secretion by prostate cancer cells. Further, we discovered that these effects were caused by inhibition of miR-20a in prostate cancer cells by pterostilbene. MiR-20a could target the 3' untranslated region (UTR) of MICA/B, resulting in their expression down-regulation. Inhibition of TGF-β1 function by its specific antibody attenuated its impairment to NKG2D on NK cells. Finally, we observed that pterostilbene-treated prostate cancer cells were more easily to be killed by NK cells. Taken together, our findings demonstrated inhibition of miR-20a by pterostilbene in prostate cancer cells could increase MICA/B expression and decrease TGF-β1 secretion, which enhanced NK cell-mediated cytotoxicity againt prostate cancer cells, suggesting a potential approach for increasing anti-prostate cancer immune.
Keywords: MICA/B; NKG2D; Natural killer cell; Prostate cancer; Pterostilbene; TGF-β1; miR-20a.
©2023PublishedbyElsevierLtd.
Conflict of interest statement
There are no potential conflicts of interest in this paper.
Figures


Similar articles
-
Ovarian tumor-associated microRNA-20a decreases natural killer cell cytotoxicity by downregulating MICA/B expression.Cell Mol Immunol. 2014 Sep;11(5):495-502. doi: 10.1038/cmi.2014.30. Epub 2014 May 12. Cell Mol Immunol. 2014. PMID: 24813230 Free PMC article.
-
miR-20a regulates sensitivity of colorectal cancer cells to NK cells by targeting MICA.Biosci Rep. 2019 Jul 2;39(7):BSR20180695. doi: 10.1042/BSR20180695. Print 2019 Jul 31. Biosci Rep. 2019. PMID: 30988067 Free PMC article.
-
Platelet-derived TGF-β1 mediates the down-modulation of NKG2D expression and may be responsible for impaired natural killer (NK) cytotoxicity in women with endometriosis.Hum Reprod. 2016 Jul;31(7):1462-74. doi: 10.1093/humrep/dew057. Epub 2016 Apr 30. Hum Reprod. 2016. PMID: 27130956
-
Leveraging NKG2D Ligands in Immuno-Oncology.Front Immunol. 2021 Jul 29;12:713158. doi: 10.3389/fimmu.2021.713158. eCollection 2021. Front Immunol. 2021. PMID: 34394116 Free PMC article. Review.
-
Novel role of immune-related non-coding RNAs as potential biomarkers regulating tumour immunoresponse via MICA/NKG2D pathway.Biomark Res. 2023 Oct 2;11(1):86. doi: 10.1186/s40364-023-00530-4. Biomark Res. 2023. PMID: 37784183 Free PMC article. Review.
References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA A Cancer J. Clin. 2021;71(1):7–33. - PubMed
-
- Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F., Redfern C.H., Ferrari A.C., Dreicer R., Sims R.B., Xu Y., Frohlich M.W., Schellhammer P.F. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363(5):411–422. - PubMed
LinkOut - more resources
Full Text Sources

