Self-Expanding Versus Balloon Expanding Coronary Stents in Intervention of the Degenerated Saphenous Vein Graft: Memmingen Coronary Artery Bypass Stenosis Trial (MECAST)
- PMID: 37064643
- PMCID: PMC10101740
- DOI: 10.1155/2023/9412132
Self-Expanding Versus Balloon Expanding Coronary Stents in Intervention of the Degenerated Saphenous Vein Graft: Memmingen Coronary Artery Bypass Stenosis Trial (MECAST)
Abstract
Objectives: The aim of this retrospective analysis was to compare the patient outcome after interventional therapy of saphenous vein graft (SVG) stenoses in an all-comers population receiving either self-expanding drug-eluting stents (SExS) or balloon expanding drug-eluting stents (BExS).
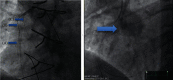
Background: The interventional therapy of degenerated SVGs remains challenging. Diameter variations of stenotic segments and friable plaques can lead to malapposition and distal embolization with increased major adverse cardiac event (MACE) rates.
Methods: 107 patients with a total of 130 SVG interventions were separated into two groups according to either SExS (n = 51) or BExS (n = 56) treatment. Primary endpoint was the MACE rate, which is defined as the composite of cardiac death, myocardial infarction (MI), target vessel (TVR), and target lesion revascularization (TLR) at 30 days and at one-year follow-up.
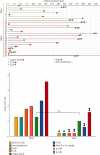
Results: Both patient groups did not differ significantly regarding patient characteristics. The patient outcome was significantly better in the SExS patient group: the MACE rate at 30 days was 1/51 (2.0%) in group SExS vs. 7/56 (12.5%) in group BExS; p < 0.05. At one-year follow-up, the MACE rate remained significantly lower in the SExS group 8/51(15.7%) vs. 20/56 (35.7%) in the BExS group, p < 0.02. Additionally, cardiac death occurred significantly later within the SExS patient group compared to the BExS group (p < 0.05). A better overall outcome of patients with de novo SVG-stenosis compared to patients with previous CABG (coronary artery bypass graft) intervention was noted in both groups.
Conclusion: Our findings demonstrate that SVG treatment with SExS is safe and provides clinical benefits by comparatively improving short and especially long-term patient outcomes.
Copyright © 2023 Marcus Siry et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- ElBardissi A. W., Aranki S. F., Sheng S., O’Brien S. M., Greenberg C. C., Gammie J. S. Trends in isolated coronary artery bypass grafting: an analysis of the society of thoracic surgeons adult cardiac surgery database. The Journal of Thoracic and Cardiovascular Surgery . 2012;143(2):273–281. doi: 10.1016/j.jtcvs.2011.10.029. - DOI - PubMed
-
- Kalan J. M., Roberts W. C. Morphologic findings in saphenous veins used as coronary arterial bypass conduits for longer than 1 year: necropsy analysis of 53 patients, 123 saphenous veins, and 1865 five-millimeter segments of veins. American Heart Journal . 1990;119(5):1164–1184. doi: 10.1016/s0002-8703(05)80249-2. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
