Bioaccumulation of CdSe Quantum Dots Show Biochemical and Oxidative Damage in Wistar Rats
- PMID: 37064800
- PMCID: PMC10101743
- DOI: 10.1155/2023/7707452
Bioaccumulation of CdSe Quantum Dots Show Biochemical and Oxidative Damage in Wistar Rats
Abstract
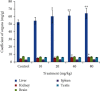
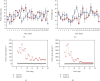
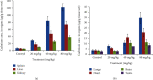
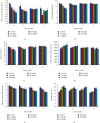
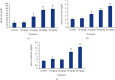
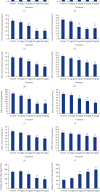
Cadmium selenium quantum dots (CdSe QDs) with modified surfaces exhibit superior dispersion stability and high fluorescence yield, making them desirable biological probes. The knowledge of cellular and biochemical toxicity has been lacking, and there is little information on the correlation between in vitro and in vivo data. The current study was carried out to assess the toxicity of CdSe QDs after intravenous injection in Wistar male rats (230 g). The rats were given a single dose of QDs of 10, 20, 40, and 80 mg/kg and were kept for 30 days. Following that, various biochemical assays, hematological parameters, and bioaccumulation studies were carried out. Functional as well as clinically significant changes were observed. There was a significant increase in WBC while the RBC decreased. This suggested that CdSe quantum dots had inflammatory effects on the treated rats. The various biochemical assays clearly showed that high dose induced hepatic injury. At a dose of 80 mg/kg, bioaccumulation studies revealed that the spleen (120 g/g), liver (78 g/g), and lungs (38 g/g) accumulated the most. In treated Wistar rats, the bioretention profile of QDs was in the following order: the spleen, liver, kidney, lungs, heart, brain, and testis. The accumulation of these QDs induced the generation of intracellular reactive oxygen species, resulting in an alteration in antioxidant activity. It is concluded that these QDs caused oxidative stress, which harmed cellular functions and, under certain conditions, caused partial brain, kidney, spleen, and liver dysfunction. This is one of the most comprehensive in vivo studies on the nanotoxicity of CdSe quantum dots.
Copyright © 2023 Kishan Das et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Thomas T. Nanotechnology in consumer products: addressing potential health and ssafety implications for consumers. In: Monteiro-Riviere N. A., Tran C. L., editors. Nanotoxicology Progress toward Nanomedicine . Boca Raton, Boca Raton: CRC Press; 2014. pp. 97–112. - DOI
-
- Joksimovic N., Selakovic D., Jovicic N., et al. Nanoplastics as an invisible threat to humans and the environment. Journal of Nanomaterials . 2022;2022(12):p. 15. doi: 10.1155/2022/6707819.6707819 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

