Vitamin D and chronic kidney disease: Insights on lipid metabolism of tubular epithelial cell and macrophages in tubulointerstitial fibrosis
- PMID: 37064892
- PMCID: PMC10090472
- DOI: 10.3389/fphys.2023.1145233
Vitamin D and chronic kidney disease: Insights on lipid metabolism of tubular epithelial cell and macrophages in tubulointerstitial fibrosis
Abstract
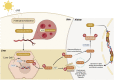
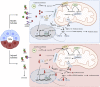
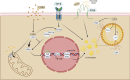
Chronic kidney disease (CKD) has been recognized as a significant global health problem due to being an important contributor to morbidity and mortality. Inflammation is the critical event that leads to CKD development orchestrated by a complex interaction between renal parenchyma and immune cells. Particularly, the crosstalk between tubular epithelial cells (TECs) and macrophages is an example of the critical cell communication in the kidney that drives kidney fibrosis, a pathological feature in CKD. Metabolism dysregulation of TECs and macrophages can be a bridge that connects inflammation and fibrogenesis. Currently, some evidence has reported how cellular lipid disturbances can affect kidney disease and cause tubulointerstitial fibrosis highlighting the importance of investigating potential molecules that can restore metabolic parameters. Vitamin D (VitD) is a hormone naturally produced by mammalian cells in a coordinated manner by the skin, liver, and kidneys. VitD deficiency or insufficiency is prevalent in patients with CKD, and serum levels of VitD are inversely correlated with the degree of kidney inflammation and renal function. Proximal TECs and macrophages produce the active form of VitD, and both express the VitD receptor (VDR) that evidence the importance of this nutrient in regulating their functions. However, whether VitD signaling drives physiological and metabolism improvement of TECs and macrophages during kidney injury is an open issue to be debated. In this review, we brought to light VitD as an important metabolic modulator of lipid metabolism in TECs and macrophages. New scientific approaches targeting VitD e VDR signaling at the cellular metabolic level can provide a better comprehension of its role in renal physiology and CKD progression.
Keywords: VDR (vitamin D receptor); beta-oxidation; immune cell; metabolism; renal fibrosis.
Copyright © 2023 Gonçalves, Andrade-Silva, Basso and Câmara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

