Inhibition of the calcium-sensing receptor by extracellular phosphate ions and by intracellular phosphorylation
- PMID: 37064904
- PMCID: PMC10102455
- DOI: 10.3389/fphys.2023.1154374
Inhibition of the calcium-sensing receptor by extracellular phosphate ions and by intracellular phosphorylation
Abstract
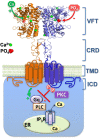
As both a sensor of extracellular calcium (Ca2+ o) concentration and a key controller of Ca2+ o homeostasis, one of the most interesting properties of the calcium-sensing receptor (CaR) is its sensitivity to, and modulation by, ions and small ligands other than Ca2+. There is emerging evidence that extracellular phosphate can act as a partial, non-competitive CaR antagonist to modulate parathyroid hormone (PTH) secretion, thus permitting the CaR to integrate mineral homeostasis more broadly. Interestingly, phosphorylation of certain intracellular CaR residues can also inhibit CaR responsiveness. Thus, negatively charged phosphate can decrease CaR activity both extracellularly (via association with arginine) and intracellularly (via covalent phosphorylation).
Keywords: calcium-sensing receptor; parathyroid hormone; phosphate-sensing; receptor phosphorylation; secondary hyperparathyroidism.
Copyright © 2023 Centeno, Binmahfouz, Alghamdi and Ward.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

