Nitric oxide augments signaling for contraction in hypoxic pulmonary arterial smooth muscle-Implications for hypoxic pulmonary hypertension
- PMID: 37064915
- PMCID: PMC10090299
- DOI: 10.3389/fphys.2023.1144574
Nitric oxide augments signaling for contraction in hypoxic pulmonary arterial smooth muscle-Implications for hypoxic pulmonary hypertension
Abstract
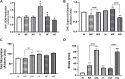
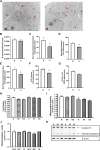
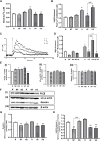
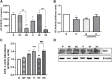
Introduction: Hypoxic persistent pulmonary hypertension in the newborn (PPHN) is usually treated with oxygen and inhaled nitric oxide (NO), both pulmonary arterial relaxants. But treatment failure with NO occurs in 25% of cases. We previously demonstrated that 72 h exposure to hypoxia, modeling PPHN, sensitized pulmonary artery smooth muscle cells (PASMC) to the contractile agonist thromboxane and inhibited relaxant adenylyl cyclase (AC) activity. Methods: In this study, we examined the effects of sodium nitroprusside (SNP), as NO donor, on the thromboxane-mediated contraction and NO-independent relaxation pathways and on reactive oxygen species (ROS) accumulation in PASMC. In addition, we examined the effect of the peroxynitrite scavenger 5,10,15,20-Tetrakis (4-sulfonatophenyl)porphyrinato Iron (III) (FeTPPS) on these processes. Results: Exposure of PASMC to 72 h hypoxia increased total intracellular ROS compared to normoxic control cells and this was mitigated by treatment of cells with either SNP or FeTPPS. Total protein nitrosylation was increased in hypoxic PASMC compared to controls. Both normoxic and hypoxic cells treated with SNP exhibited increased total protein nitrosylation and intracellular nitrite; this was reduced by treatment with FeTPPS. While cell viability and mitochondrial number were unchanged by hypoxia, mitochondrial activity was decreased compared to controls; addition of FeTPPS did not alter this. Basal and maximal mitochondrial metabolism and ATP turnover were reduced in hypoxic PASMC compared to controls. Hypoxic PASMC had higher basal Ca2+, and a heightened peak Ca2+ response to thromboxane challenge compared to controls. Addition of SNP further elevated the peak Ca2+ response, while addition of FeTPPS brought peak Ca2+ response down to control levels. AC mediated relaxation was impaired in hypoxic PASMC compared to controls but was normalized following treatment with FeTPPS. Addition of SNP inhibited adenylyl cyclase activity in both normoxic and hypoxic PASMC. Moreover, addition of the Ca2+ chelator BAPTA improved AC activity, but the effect was minimal. Discussion: We conclude that NO independently augments contraction and inhibits relaxation pathways in hypoxic PASMC, in part by a mechanism involving nitrogen radical formation and protein nitrosylation. These observations may partially explain impaired effectiveness of NO when treating hypoxic pulmonary hypertension.
Keywords: adenylyl cyclase; calcium; hypoxia; nitric oxide; persistent pulmonary hypertension of the newborn; smooth muscle; thromboxane.
Copyright © 2023 Hinton, Thliveris, Hatch and Dakshinamurti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Hypoxia inhibits adenylyl cyclase catalytic activity in a porcine model of persistent pulmonary hypertension of the newborn.Am J Physiol Lung Cell Mol Physiol. 2018 Dec 1;315(6):L933-L944. doi: 10.1152/ajplung.00130.2018. Epub 2018 Sep 20. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 30234376
-
Hypoxia induces hypersensitivity and hyperreactivity to thromboxane receptor agonist in neonatal pulmonary arterial myocytes.Am J Physiol Lung Cell Mol Physiol. 2006 Feb;290(2):L375-84. doi: 10.1152/ajplung.00307.2005. Epub 2005 Oct 7. Am J Physiol Lung Cell Mol Physiol. 2006. PMID: 16214814
-
Pulmonary artery smooth muscle cell [Ca2+]i and contraction: responses to diphenyleneiodonium and hypoxia.Am J Physiol. 1997 Sep;273(3 Pt 1):L603-11. doi: 10.1152/ajplung.1997.273.3.L603. Am J Physiol. 1997. PMID: 9316495
-
Nitric oxide modulates hypoxic pulmonary smooth muscle cell proliferation and apoptosis by regulating carbon monoxide pathway.Acta Pharmacol Sin. 2007 Jan;28(1):28-35. doi: 10.1111/j.1745-7254.2007.00483.x. Acta Pharmacol Sin. 2007. PMID: 17184579
-
The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension.Microcirculation. 2006 Dec;13(8):615-32. doi: 10.1080/10739680600930222. Microcirculation. 2006. PMID: 17085423 Review.
Cited by
-
Key Therapeutic Targets to Treat Hyperglycemia-Induced Atherosclerosis Analyzed Using a Petri Net-Based Model.Metabolites. 2023 Dec 8;13(12):1191. doi: 10.3390/metabo13121191. Metabolites. 2023. PMID: 38132873 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

