Altered coronary artery function, arteriogenesis and endothelial YAP signaling in postnatal hypertrophic cardiomyopathy
- PMID: 37064918
- PMCID: PMC10102353
- DOI: 10.3389/fphys.2023.1136852
Altered coronary artery function, arteriogenesis and endothelial YAP signaling in postnatal hypertrophic cardiomyopathy
Abstract
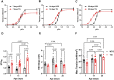
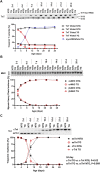
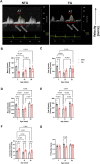
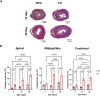
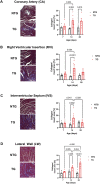
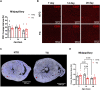
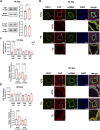
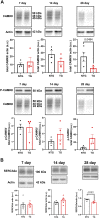
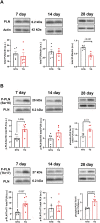
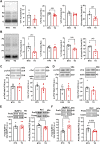
Introduction: Hypertrophic cardiomyopathy (HCM) is a cardiovascular genetic disease caused largely by sarcomere protein mutations. Gaps in our understanding exist as to how maladaptive sarcomeric biophysical signals are transduced to intra- and extracellular compartments leading to HCM progression. To investigate early HCM progression, we focused on the onset of myofilament dysfunction during neonatal development and examined cardiac dynamics, coronary vascular structure and function, and mechano-transduction signaling in mice harboring a thin-filament HCM mutation. Methods: We studied postnatal days 7-28 (P7-P28) in transgenic (TG) TG-cTnT-R92Q and non-transgenic (NTG) mice using skinned fiber mechanics, echocardiography, biochemistry, histology, and immunohistochemistry. Results: At P7, skinned myofiber bundles exhibited an increased Ca2+-sensitivity (pCa50 TG: 5.97 ± 0.04, NTG: 5.84 ± 0.01) resulting from cTnT-R92Q expression on a background of slow skeletal (fetal) troponin I and α/β myosin heavy chain isoform expression. Despite the transition to adult isoform expressions between P7-P14, the increased Ca2+- sensitivity persisted through P28 with no apparent differences in gross morphology among TG and NTG hearts. At P7 significant diastolic dysfunction was accompanied by coronary flow perturbation (mean diastolic velocity, TG: 222.5 ± 18.81 mm/s, NTG: 338.7 ± 28.07 mm/s) along with localized fibrosis (TG: 4.36% ± 0.44%, NTG: 2.53% ± 0.47%). Increased phosphorylation of phospholamban (PLN) was also evident indicating abnormalities in Ca2+ homeostasis. By P14 there was a decline in arteriolar cross-sectional area along with an expansion of fibrosis (TG: 9.72% ± 0.73%, NTG: 2.72% ± 0.2%). In comparing mechano-transduction signaling in the coronary arteries, we uncovered an increase in endothelial YAP expression with a decrease in its nuclear to cytosolic ratio at P14 in TG hearts, which was reversed by P28. Conclusion: We conclude that those early mechanisms that presage hypertrophic remodeling in HCM include defective biophysical signals within the sarcomere that drive diastolic dysfunction, impacting coronary flow dynamics, defective arteriogenesis and fibrosis. Changes in mechano-transduction signaling between the different cellular compartments contribute to the pathogenesis of HCM.
Keywords: YAP signaling; coronary flow; echocardiography; fibrosis; hypertrophic cardiomyopathy; mechano-signaling.
Copyright © 2023 Langa, Marszalek, Warren, Chowdhury, Halas, Batra, Rafael-Clyke, Bacon, Goldspink, Solaro and Wolska.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Reduction in myofilament Ca2+ sensitivity partially ameliorates the cardiac phenotype in hypertrophic cardiomyopathy linked to a TnT-R92Q mutation.Front Physiol. 2025 May 23;16:1600117. doi: 10.3389/fphys.2025.1600117. eCollection 2025. Front Physiol. 2025. PMID: 40488145 Free PMC article.
-
Mechano-energetic uncoupling in hypertrophic cardiomyopathy: Pathophysiological mechanisms and therapeutic opportunities.J Mol Cell Cardiol Plus. 2023 May 6;4:100036. doi: 10.1016/j.jmccpl.2023.100036. eCollection 2023 Jun. J Mol Cell Cardiol Plus. 2023. PMID: 39801694 Free PMC article. Review.
-
Modifications of Sarcoplasmic Reticulum Function Prevent Progression of Sarcomere-Linked Hypertrophic Cardiomyopathy Despite a Persistent Increase in Myofilament Calcium Response.Front Physiol. 2020 Mar 10;11:107. doi: 10.3389/fphys.2020.00107. eCollection 2020. Front Physiol. 2020. PMID: 32210830 Free PMC article.
-
Effects of Sarcomere Activators and Inhibitors Targeting Myosin Cross-Bridges on Ca2+-Activation of Mature and Immature Mouse Cardiac Myofilaments.Mol Pharmacol. 2022 May;101(5):286-299. doi: 10.1124/molpharm.121.000420. Epub 2022 Mar 2. Mol Pharmacol. 2022. PMID: 35236770 Free PMC article.
-
Cardiac Troponin and Tropomyosin: Structural and Cellular Perspectives to Unveil the Hypertrophic Cardiomyopathy Phenotype.Front Physiol. 2016 Sep 23;7:429. doi: 10.3389/fphys.2016.00429. eCollection 2016. Front Physiol. 2016. PMID: 27721798 Free PMC article. Review.
Cited by
-
Reduction in myofilament Ca2+ sensitivity partially ameliorates the cardiac phenotype in hypertrophic cardiomyopathy linked to a TnT-R92Q mutation.Front Physiol. 2025 May 23;16:1600117. doi: 10.3389/fphys.2025.1600117. eCollection 2025. Front Physiol. 2025. PMID: 40488145 Free PMC article.
-
Force-sensing protein expression in response to cardiovascular mechanotransduction.EBioMedicine. 2024 Dec;110:105412. doi: 10.1016/j.ebiom.2024.105412. Epub 2024 Oct 30. EBioMedicine. 2024. PMID: 39481337 Free PMC article. Review.
-
Mechano-energetic uncoupling in hypertrophic cardiomyopathy: Pathophysiological mechanisms and therapeutic opportunities.J Mol Cell Cardiol Plus. 2023 May 6;4:100036. doi: 10.1016/j.jmccpl.2023.100036. eCollection 2023 Jun. J Mol Cell Cardiol Plus. 2023. PMID: 39801694 Free PMC article. Review.
References
-
- Alves M. L., Dias F. a. L., Gaffin R. D., Simon J. N., Montminy E. M., Biesiadecki B. J., et al. (2014). Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ. Cardiovasc Genet. 7, 132–143. 10.1161/CIRCGENETICS.113.000324 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

