Cytotoxicity and Antibacterial Potentials of Mixed Ligand Cu(II) and Zn(II) Complexes: A Combined Experimental and Computational Study
- PMID: 37065050
- PMCID: PMC10099420
- DOI: 10.1021/acsomega.3c00916
Cytotoxicity and Antibacterial Potentials of Mixed Ligand Cu(II) and Zn(II) Complexes: A Combined Experimental and Computational Study
Abstract
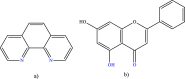
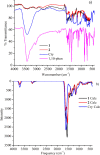
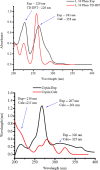
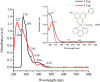
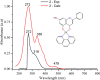
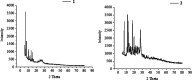
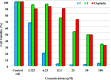
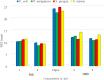
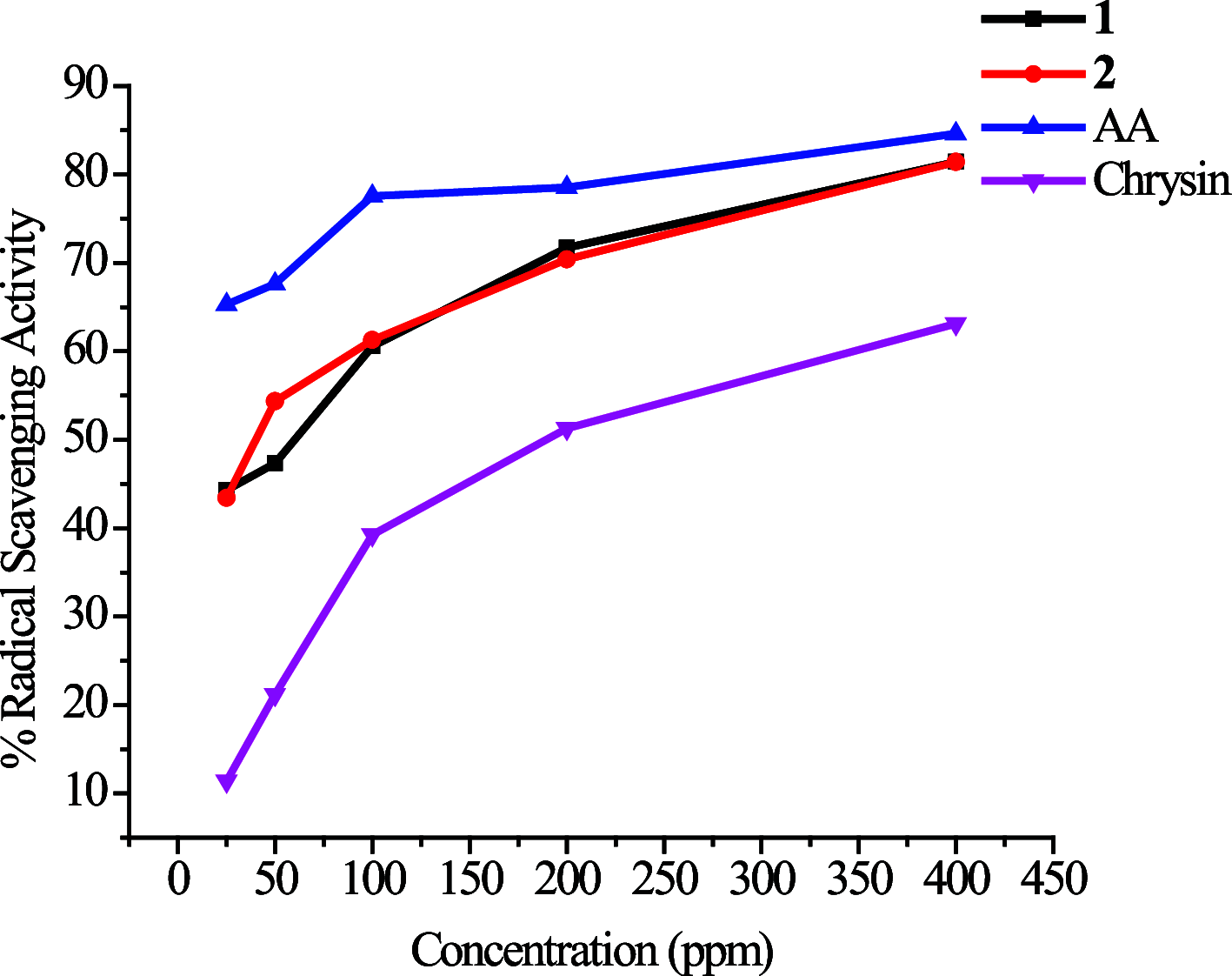
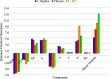
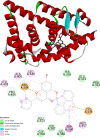
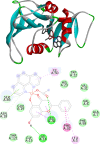
[Cu(C15H9O4)(C12H8N2)O2C2H3]·3H2O (1) and [Zn(C15H9O4)(C12H8N2)]O2C2H3 (2) have been synthesized and characterized by ultraviolet-visible (UV-vis) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, mass spectrometry, thermogravimetric analysis/differential thermal analysis (TGA/DTA), X-ray diffraction (XRD), scanning electron microscopy-energy-dispersive X-ray spectroscopy (SEM-EDX), and molar conductance, and supported by density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations. Square pyramidal and tetrahedral geometries are proposed for Cu(II) and Zn(II) complexes, respectively, and the XRD patterns showed the polycrystalline nature of the complexes. Furthermore, in vitro cytotoxic activity of the complexes was evaluated against the human breast cancer cell line (MCF-7). A Cu(II) centered complex with an IC50 value of 4.09 μM was more effective than the Zn(II) centered complex and positive control, cisplatin, which displayed IC50 values of 75.78 and 18.62 μM, respectively. In addition, the newly synthesized complexes experienced the innate antioxidant nature of the metal centers for scavenging the DPPH free radical (up to 81% at 400 ppm). The biological significance of the metal complexes was inferred from the highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) energy band gap, which was found to be 2.784 and 3.333 eV, respectively for 1 and 2, compared to the ligands, 1,10-phenathroline (4.755 eV) and chrysin (4.403 eV). Moreover, the molecular docking simulations against estrogen receptor alpha (ERα; PDB: 5GS4) were strongly associated with the in vitro biological activity results (E B and K i are -8.35 kcal/mol and 0.76 μM for 1, -7.52 kcal/mol and 3.07 μM for 2, and -6.32 kcal/mol and 23.42 μM for cisplatin). However, more research on in vivo cytotoxicity is suggested to confirm the promising cytotoxicity results.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Karges J.; Stokes R. W.; Cohen S. M. Metal complexes for therapeutic applications. Trends Chem. 2021, 3, 523–534. 10.1016/j.trechm.2021.03.006. - DOI - PMC - PubMed
- Amarsy I.; Papot S.; Gasser G. Stimuli-Responsive Metal Complexes for Biomedical Applications. Angew. Chem., Int. Ed. 2022, 134, e20220590010.1002/ange.202205900. - DOI - PubMed
-
- Kilpin K. J.; Dyson P. J. Enzyme inhibition by metal complexes: concepts, strategies and applications. Chem. Sci. 2013, 4, 1410–1419. 10.1039/c3sc22349c. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

