Integrative Genomic Analysis of m6a-SNPs Identifies Potential Functional Variants Associated with Alzheimer's Disease
- PMID: 37065064
- PMCID: PMC10099436
- DOI: 10.1021/acsomega.3c00696
Integrative Genomic Analysis of m6a-SNPs Identifies Potential Functional Variants Associated with Alzheimer's Disease
Abstract
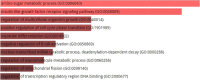
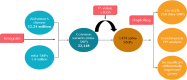
Alzheimer's disease (AD) is a neurodegenerative disorder that affects 35 million people worldwide. However, no potential therapeutics currently are available for AD because of the multiple factors involved in it, such as regulatory factors with their candidate genes, factors associated with the expression levels of its corresponding genes, and many others. To date, 29 novel loci from GWAS have been reported for AD by the Psychiatric Genomics Consortium (PGC2). Nevertheless, the main challenge of the post-GWAS era, namely to detect significant variants of the target disease, has not been conducted for AD. N6-methyladenosine (m6a) is reported as the most prevalent mRNA modification that exists in eukaryotes and that influences mRNA nuclear export, translation, splicing, and the stability of mRNA. Furthermore, studies have also reported m6a's association with neurogenesis and brain development. We carried out an integrative genomic analysis of AD variants from GWAS and m6a-SNPs from m6AVAR to identify the effects of m6a-SNPs on AD and identified the significant variants using the statistically significance value (p-value <0.05). The cis-regularity variants with their corresponding genes and their influence on gene expression in the gene expression profiles of AD patients were determined, and showed 1458 potential m6a-SNPs (based on p-value <0.05) associated with AD. eQTL analysis showed that 258 m6a-SNPs had cis-eQTL signals that overlapped with six significant differentially expressed genes based on p-value <0.05 in two datasets of AD gene expression profiles. A follow-up study to elucidate the impact of our identified m6a-SNPs in the experimental study would validate our findings for AD, which would contribute to the etiology of AD.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Integrative approaches to m6A and m5C RNA modifications in autism spectrum disorder revealing potential causal variants.Mamm Genome. 2025 Mar;36(1):280-292. doi: 10.1007/s00335-024-10095-8. Epub 2024 Dec 30. Mamm Genome. 2025. PMID: 39738578
-
Genome-wide detection of m6A-associated SNPs in atrial fibrillation pathogenesis.Front Cardiovasc Med. 2023 May 26;10:1152851. doi: 10.3389/fcvm.2023.1152851. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37304952 Free PMC article.
-
Dynamics of N6-methyladenosine modification during Alzheimer's disease development.Heliyon. 2024 Feb 25;10(6):e26911. doi: 10.1016/j.heliyon.2024.e26911. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38496847 Free PMC article.
-
Shared genetic etiology underlying Alzheimer's disease and type 2 diabetes.Mol Aspects Med. 2015 Jun-Oct;43-44:66-76. doi: 10.1016/j.mam.2015.06.006. Epub 2015 Jun 23. Mol Aspects Med. 2015. PMID: 26116273 Free PMC article. Review.
-
Reversible N6-methyladenosine of RNA: The regulatory mechanisms on gene expression and implications in physiology and pathology.Genes Dis. 2020 Jul 6;7(4):585-597. doi: 10.1016/j.gendis.2020.06.011. eCollection 2020 Dec. Genes Dis. 2020. PMID: 33335958 Free PMC article. Review.
Cited by
-
Regulatory roles of N6-methyladenosine (m6A) methylation in RNA processing and non-communicable diseases.Cancer Gene Ther. 2024 Oct;31(10):1439-1453. doi: 10.1038/s41417-024-00789-1. Epub 2024 Jun 5. Cancer Gene Ther. 2024. PMID: 38839892 Review.
-
Computational Approaches to Evaluate the Acetylcholinesterase Binding Interaction with Taxifolin for the Management of Alzheimer's Disease.Molecules. 2024 Jan 31;29(3):674. doi: 10.3390/molecules29030674. Molecules. 2024. PMID: 38338420 Free PMC article.
References
-
- Elmaidomy A. H.; Abdelmohsen U. R.; Alsenani F.; Aly H. F.; Shams S. G. E.; Younis E. A.; Ahmed K. A.; Sayed A. M.; Owis A. I.; Afifi N.; El Amir D. The Anti-Alzheimer Potential of Tamarindus Indica: An in Vivo Investigation Supported by in Vitro and in Silico Approaches. RSC Adv. 2022, 12, 11769–11785. 10.1039/D2RA01340A. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources