Impact of porcine circovirus type 2 on porcine epidemic diarrhea virus replication in the IPI-FX cell line depends on the order of infection
- PMID: 37065133
- PMCID: PMC10100733
- DOI: 10.3389/fmicb.2023.1162104
Impact of porcine circovirus type 2 on porcine epidemic diarrhea virus replication in the IPI-FX cell line depends on the order of infection
Abstract
Introduction: A study in 2006 showed that the clinical course of PEDV disease was markedly aggravated by transplacental infection of PCV2. Therefore, we investigated whether the small intestine supports PCV2 replication and the effect of PCV2 infection on PEDV replication in epithelial cells in vitro.
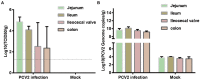
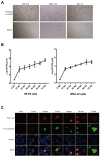
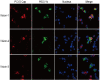
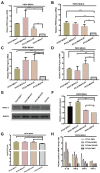
Methods: To confirm the intestinal tropism of PCV2, the viral loads in the small-intestinal tissues after PCV2 infection were determined with virus titration, and the viral titers in the infected pig jejunum, ileum, ileocecal valve, and colon were 104.86, 104.09, 102.52, and 102.35 TCID50/g, respectively. We then determined the propagation characteristics of PCV2 in ileal epithelial cells (IPI-FX) and jejunal epithelial cells (IPEC-J2) with an immunoperoxidase monolayer assay, virus titration, and an immunofluorescence assay. Both IPI-FX and IPEC-J2 cells supported the replication of PCV2, with titers of 105.5 and 105.0 TCID50/ml, respectively. We established an infection model of PCV2 and PEDV in IPI-FX cells and found that PEDV and PCV2 infected the cells individually and together. The effects of PCV2 infection on PEDV replication were determined with reverse transcription-quantitative PCR (qPCR), western blotting, and virus titration. When PCV2 infected IPI-FX cells before PEDV, PCV2 significantly inhibited the replication of PEDV in a dose- and time-dependent manner and that the mRNAs of IFN-β, TNF-α, IL1β, and OASL were downregulated (detected with qPCR). Surprisingly, when IPI-FX cells were co-infected with PCV2 and PEDV, PCV2 promoted the replication of PEDV, the expression of the host IFN-β, TNF-α, IL1β, and OASL mRNAs was upregulated.
Discussion: These findings demonstrate that the co-infection of IPI-FX cells with PCV2 and PEDV represents an excellent in vitro model in which to investigate their combined pathogenic mechanisms.
Keywords: IPEC-J2 cells; IPI-FX cells; co-infection; porcine circovirus type 2; porcine epidemic diarrhea virus.
Copyright © 2023 Zhang, Shi, Wei, Shi, Cao, Liu, Liu, Li, Liu, Feng and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Infection of porcine circovirus 2 (PCV2) in intestinal porcine epithelial cell line (IPEC-J2) and interaction between PCV2 and IPEC-J2 microfilaments.Virol J. 2014 Nov 19;11:193. doi: 10.1186/s12985-014-0193-0. Virol J. 2014. PMID: 25407967 Free PMC article.
-
Adenovirus vector-mediated single chain variable fragments target the nucleocapsid protein of porcine epidemic diarrhea virus and protect against viral infection in piglets.Front Immunol. 2023 Jan 24;14:1058327. doi: 10.3389/fimmu.2023.1058327. eCollection 2023. Front Immunol. 2023. PMID: 36761768 Free PMC article.
-
The effects of transplacental porcine circovirus type 2 infection on porcine epidemic diarrhoea virus-induced enteritis in preweaning piglets.Vet J. 2006 May;171(3):445-50. doi: 10.1016/j.tvjl.2005.02.016. Vet J. 2006. PMID: 16624710 Free PMC article.
-
Susceptibility of porcine IPI-2I intestinal epithelial cells to infection with swine enteric coronaviruses.Vet Microbiol. 2019 Jun;233:21-27. doi: 10.1016/j.vetmic.2019.04.014. Epub 2019 Apr 14. Vet Microbiol. 2019. PMID: 31176408 Free PMC article.
-
IFN-lambda preferably inhibits PEDV infection of porcine intestinal epithelial cells compared with IFN-alpha.Antiviral Res. 2017 Apr;140:76-82. doi: 10.1016/j.antiviral.2017.01.012. Epub 2017 Jan 19. Antiviral Res. 2017. PMID: 28109912 Free PMC article.
Cited by
-
Genomic composition and pathomechanisms of porcine circoviruses: A review.Virulence. 2024 Dec;15(1):2439524. doi: 10.1080/21505594.2024.2439524. Epub 2024 Dec 11. Virulence. 2024. PMID: 39662970 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

