Coinfection with influenza virus and non-typeable Haemophilus influenzae aggregates inflammatory lung injury and alters gut microbiota in COPD mice
- PMID: 37065141
- PMCID: PMC10098174
- DOI: 10.3389/fmicb.2023.1137369
Coinfection with influenza virus and non-typeable Haemophilus influenzae aggregates inflammatory lung injury and alters gut microbiota in COPD mice
Abstract
Background: Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is associated with high mortality rates. Viral and bacterial coinfection is the primary cause of AECOPD. How coinfection with these microbes influences host inflammatory response and the gut microbiota composition is not entirely understood.
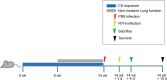
Methods: We developed a mouse model of AECOPD by cigarette smoke exposure and sequential infection with influenza H1N1 virus and non-typeable Haemophilus influenzae (NTHi). Viral and bacterial titer was determined using MDCK cells and chocolate agar plates, respectively. The levels of cytokines, adhesion molecules, and inflammatory cells in the lungs were measured using Bio-Plex and flow cytometry assays. Gut microbiota was analyzed using 16S rRNA gene sequencing. Correlations between cytokines and gut microbiota were determined using Spearman's rank correlation coefficient test.
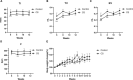
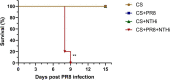
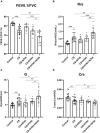
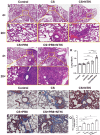
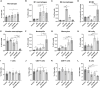
Results: Coinfection with H1N1 and NTHi resulted in more severe lung injury, higher mortality, declined lung function in COPD mice. H1N1 enhanced NTHi growth in the lungs, but NTHi had no effect on H1N1. In addition, coinfection increased the levels of cytokines and adhesion molecules, as well as immune cells including total and M1 macrophages, neutrophils, monocytes, NK cells, and CD4 + T cells. In contrast, alveolar macrophages were depleted. Furthermore, coinfection caused a decline in the diversity of gut bacteria. Muribaculaceae, Lactobacillus, Akkermansia, Lachnospiraceae, and Rikenella were further found to be negatively correlated with cytokine levels, whereas Bacteroides was positively correlated.
Conclusion: Coinfection with H1N1 and NTHi causes a deterioration in COPD mice due to increased lung inflammation, which is correlated with dysbiosis of the gut microbiota.
Keywords: COPD; inflammation; influenza; influenzae; mice; microbiota.
Copyright © 2023 Wu, Li, Lin, Xiao, Liu, Mai, Zhou, Zeng, Cheng, Weng, Zhao, Chen, Jiang, Chen, Deng, Xie, Yang, Xia and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae.Front Immunol. 2018 Nov 5;9:2530. doi: 10.3389/fimmu.2018.02530. eCollection 2018. Front Immunol. 2018. PMID: 30455693 Free PMC article. Review.
-
Correlation of adhesion molecules and non-typeable haemophilus influenzae growth in a mice coinfected model of acute inflammation.Microbes Infect. 2021 Sep-Oct;23(8):104839. doi: 10.1016/j.micinf.2021.104839. Epub 2021 May 21. Microbes Infect. 2021. PMID: 34023525
-
Contribution of dipeptidyl peptidase 4 to non-typeable Haemophilus influenzae-induced lung inflammation in COPD.Clin Sci (Lond). 2021 Sep 17;135(17):2067-2083. doi: 10.1042/CS20210099. Clin Sci (Lond). 2021. PMID: 34405230
-
The Role of Non-Typeable Haemophilus influenzae Biofilms in Chronic Obstructive Pulmonary Disease.Front Cell Infect Microbiol. 2021 Aug 4;11:720742. doi: 10.3389/fcimb.2021.720742. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34422683 Free PMC article. Review.
-
Altered Respiratory Microbiomes, Plasma Metabolites, and Immune Responses in Influenza A Virus and Methicillin-Resistant Staphylococcus aureus Coinfection.Microbiol Spectr. 2023 Aug 17;11(4):e0524722. doi: 10.1128/spectrum.05247-22. Epub 2023 Jun 15. Microbiol Spectr. 2023. PMID: 37318361 Free PMC article.
Cited by
-
The Role of Beneficial Microbiota in COVID-19: Insights from Key Bacterial Genera.Microorganisms. 2025 Apr 29;13(5):1029. doi: 10.3390/microorganisms13051029. Microorganisms. 2025. PMID: 40431202 Free PMC article. Review.
-
Effect of Mixed Probiotics on Alleviating H1N1 Influenza Infection and Regulating Gut Microbiota.Foods. 2024 Sep 27;13(19):3079. doi: 10.3390/foods13193079. Foods. 2024. PMID: 39410114 Free PMC article.
-
Lactobacillus reuteri Alleviates Hyperoxia-Induced BPD by Activating IL-22/STAT3 Signaling Pathway in Neonatal Mice.Mediators Inflamm. 2024 Dec 9;2024:4965271. doi: 10.1155/mi/4965271. eCollection 2024. Mediators Inflamm. 2024. PMID: 39687635 Free PMC article.
-
Potential of gut microbiota metabolites in treating COPD: network pharmacology and Mendelian randomization approaches.Front Microbiol. 2024 Nov 25;15:1416651. doi: 10.3389/fmicb.2024.1416651. eCollection 2024. Front Microbiol. 2024. PMID: 39654679 Free PMC article.
-
Role of Nedd4L in Macrophage Pro-Inflammatory Polarization Induced by Influenza A Virus and Lipopolysaccharide Stimulation.Microorganisms. 2024 Jun 25;12(7):1291. doi: 10.3390/microorganisms12071291. Microorganisms. 2024. PMID: 39065060 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

