Protection from successive Omicron variants with SARS-CoV-2 vaccine and monoclonal antibodies in kidney transplant recipients
- PMID: 37065151
- PMCID: PMC10095161
- DOI: 10.3389/fmicb.2023.1147455
Protection from successive Omicron variants with SARS-CoV-2 vaccine and monoclonal antibodies in kidney transplant recipients
Abstract
Introduction: Kidney transplant recipients (KTRs) are at high risk of severe COVID-19, even when they are fully vaccinated. Additional booster vaccinations or passive immunization with prophylactic monoclonal antibodies are recommended to increase their protection against severe COVID-19.
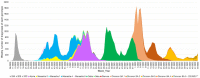
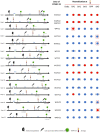
Methods: Here, we describe the neutralization of SARS-CoV-2 Delta, Omicron BA.1, BA.2, BA.4, and BA.5 variants, firstly by 39 serum samples from vaccinated KTRs exhibiting anti-spike antibody concentrations ≥264 binding antibody units (BAU)/mL and, secondly, by tixagevimab/cilgavimab.
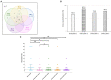
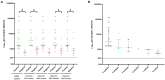
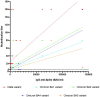
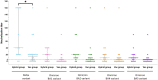
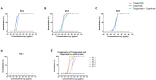
Results: No neutralization was observed for 18% of the KTRs, while serum from only 46% of patients could neutralize the five variants. Cross-neutralization of the Delta and Omicron variants occurred for 65-87% of sera samples. The anti-spike antibody concentration correlated with neutralization activity for all the variants. The neutralization titers against the Delta variant were higher in vaccinated KTRs who had previously presented with COVID-19, compared to those KTRs who had only been vaccinated. Breakthrough infections occurred in 39% of the KTRs after the study. Tixagevimab/cilgavimab poorly neutralizes Omicron variants, particularly BA.5, and does not neutralize BQ.1, which is currently the most prevalent strain.
Discussion: As a result, sera from seropositive vaccinated KTRs had poor neutralization of the successive Omicron variants. Several Omicron variants are able to escape tixagevimab/cilgavimab.
Keywords: COVID-19; SARS-CoV-2; cilgavimab; immunocompromized; kidney transplantation; neutralization; tixagevimab; vaccine.
Copyright © 2023 Moal, Valade, Boschi, Robert, Orain, Bancod, Edouard, Colson and La Scola.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Al Jurdi A., Morena L., Cote M., Bethea E., Azzi J., Riella L. V. (2022). Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am. J. Transplant. 22, 3130–3136. doi: 10.1111/ajt.17128 - DOI - PMC - PubMed
-
- Bekliz M., Adea K., Vetter P., Eberhardt C. S., Hosszu-Fellous K., Vu D. L., et al. (2022). Neutralization capacity of antibodies elicited through homologous or heterologous infection or vaccination against SARS-CoV-2 VOCs. Nat. Commun. 13:3840. doi: 10.1038/s41467-022-31556-1, PMID: - DOI - PMC - PubMed
-
- Benning L., Morath C., Bartenschlager M., Kim H., Reineke M., BeimLer J., et al. (2022). Neutralizing antibody response against the B.1.617.2 (Delta) and the B.1.1.529 (omicron) variants after a third MRNA SARS-CoV-2 vaccine dose in kidney transplant recipients. Am. J. Transplant. 22, 1873–1883. doi: 10.1111/ajt.17054, PMID: - DOI - PMC - PubMed
-
- Benotmane I., Bruel T., Planas D., Fafi-Kremer S., Schwartz O., Caillard S. (2022). A fourth dose of the MRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the Delta variant in kidney transplant recipients. Kidney Int. 101, 1073–1076. doi: 10.1016/j.kint.2022.02.011, PMID: - DOI - PMC - PubMed
-
- Benotmane I., Gautier-Vargas G., Cognard N., Olagne J., Heibel F., Braun-Parvez L., et al. (2021). Low immunization rates among kidney transplant recipients who received 2 doses of the MRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 99, 1498–1500. doi: 10.1016/j.kint.2021.04.005, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

