Serum HBsAg and HBcrAg is associated with inflammation in HBeAg-positive chronic hepatitis B patients
- PMID: 37065191
- PMCID: PMC10102387
- DOI: 10.3389/fcimb.2023.1083912
Serum HBsAg and HBcrAg is associated with inflammation in HBeAg-positive chronic hepatitis B patients
Abstract
Backgrounds & aims: Liver inflammation is the main risk factor for developing liver fibrosis, cirrhosis, and even hepatocellular carcinoma in chronic hepatitis B (CHB) patients. To replace biopsy, additional non-invasive biomarkers to diagnose and grade liver necroinflammation are urgently required in clinical practice.
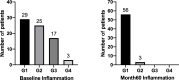
Method: Ninety-four CHB patients, including 74 HBeAg-positive and 20 HBeAg-negative patients, were enrolled and started entecavir or adefovir therapy. Serum HBV RNA, HBV DNA, HBsAg, hepatitis B core-related antigen (HBcrAg), ALT and AST levels, as well as intrahepatic HBV DNA and cccDNA were measured at baseline and during treatment. Liver inflammation was assessed at baseline and month 60 by liver biopsy. Inflammation regression was defined as a ≥1-grade decrease according to the Scheuer scoring system.
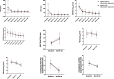
Results: In HBeAg-positive CHB patients, at baseline, serum HBsAg and HBcrAg levels negatively correlated with inflammation grade, while ALT and AST levels positively correlated with inflammation grade. AST plus HBsAg exhibited excellent diagnostic ability for significant inflammation with an AUROC of 0.896. After 60 months of antiviral treatment, almost all the patients' liver inflammation ameliorated to G1, and no patients had inflammation progression.
Conclusion: Besides ALT and AST, serum HBsAg and HBcrAg correlated with inflammation grade in HBeAg-positive CHB patients before NAs treatment. Moreover, the combination of HBsAg and AST exhibited excellent diagnostic ability for significant inflammation.
Keywords: HBcrAg; HBsAg; chronic hepatitis B; inflammation; nucleos(t)ide analogues.
Copyright © 2023 Zhao, Bian, Liao, Wang, Ren, Jiang, Liu, Chen, Hu, Duan, Lu and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Dufour D. R., Lott J. A., Nolte F. S., Gretch D. R., Koff R. S., Seeff L. B. (2000). Diagnosis and monitoring of hepatic injury. II. recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin. Chem. 46 (12), 2050–2068. doi: 10.1093/clinchem/46.12.2050 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

