Vancomycin efficiency and safety of a dosage of 40-60 mg/kg/d and corresponding trough concentrations in children with Gram-positive bacterial sepsis
- PMID: 37065209
- PMCID: PMC10098341
- DOI: 10.3389/fcimb.2023.1117717
Vancomycin efficiency and safety of a dosage of 40-60 mg/kg/d and corresponding trough concentrations in children with Gram-positive bacterial sepsis
Abstract
Background: Optimal vancomycin trough concentrations and dosages remain controversial in sepsis children. We aim to investigate vancomycin treatment outcomes with a dosage of 40-60 mg/kg/d and corresponding trough concentrations in children with Gram-positive bacterial sepsis from a clinical perspective.
Methods: Children diagnosed with Gram-positive bacterial sepsis and received intravenous vancomycin therapy between January 2017 and June 2020 were enrolled retrospectively. Patients were categorized as success and failure groups according to treatment outcomes. Laboratory, microbiological, and clinical data were collected. The risk factors for treatment failure were analyzed by logistic regression.
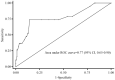
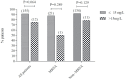
Results: In total, 186 children were included, of whom 167 (89.8%) were enrolled in the success group and 19 (10.2%) in the failure group. The initial and mean vancomycin daily doses in failure group were significantly higher than those in success group [56.9 (IQR =42.1-60.0) vs. 40.5 (IQR =40.0-57.1), P=0.016; 57.0 (IQR =45.8-60.0) vs. 50.0 (IQR =40.0-57.6) mg/kg/d, P=0.012, respectively] and median vancomycin trough concentrations were similar between two groups [6.9 (4.0-12.1) vs.7.3 (4.5-10.6) mg/L, P=0.568)]. Moreover, there was no significant differences in treatment success rate between vancomycin trough concentrations ≤15 mg/L and >15 mg/L (91.2% vs. 75.0%, P=0.064). No vancomycin-related nephrotoxicity adverse effects occurred among all enrolled patients. Multivariate analysis revealed that a PRISM III score ≥10 (OR =15.011; 95% CI: 3.937-57.230; P<0.001) was the only independent clinical factor associated with increased incidence of treatment failure.
Conclusions: Vancomycin dosages of 40-60 mg/kg/d are effective and have no vancomycin-related nephrotoxicity adverse effects in children with Gram-positive bacterial sepsis. Vancomycin trough concentrations >15 mg/L are not an essential target for these Gram-positive bacterial sepsis patients. PRISM III scores ≥10 may serve as an independent risk factor for vancomycin treatment failure in these patients.
Keywords: Gram-positive bacterial; children; dosages; tough concentrations; vancomycin.
Copyright © 2023 Peng, Guo, Zhang, Tian, Gu, Li, Li and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Retrospective analysis of vancomycin treatment outcomes in Chinese paediatric patients with suspected Gram-positive infection.J Clin Pharm Ther. 2016 Dec;41(6):650-656. doi: 10.1111/jcpt.12437. Epub 2016 Aug 31. J Clin Pharm Ther. 2016. PMID: 27578443
-
[Clinical efficacy and safety of vancomycin compared with linezolid for the treatment of neonatal gram-positive bacterial sepsis].Zhonghua Er Ke Za Zhi. 2016 Sep;54(9):686-91. doi: 10.3760/cma.j.issn.0578-1310.2016.09.011. Zhonghua Er Ke Za Zhi. 2016. PMID: 27596084 Chinese.
-
Effect of low vs. high vancomycin trough level on the clinical outcomes of adult patients with sepsis or gram-positive bacterial infections: a systematic review and meta-analysis.BMC Infect Dis. 2024 Oct 7;24(1):1114. doi: 10.1186/s12879-024-09927-4. BMC Infect Dis. 2024. PMID: 39375599 Free PMC article.
-
Efficacy of Vancomycin on Gram-Positive Bacterial Infection in Elderly Critical Patients and Risk Factors Associated With Nephrotoxicity.Arch Iran Med. 2018 Aug 1;21(8):349-355. Arch Iran Med. 2018. PMID: 30113856
-
Systematic review and meta-analysis to explore optimal therapeutic range of vancomycin trough level for infected paediatric patients with Gram-positive pathogens to reduce mortality and nephrotoxicity risk.Int J Antimicrob Agents. 2021 Aug;58(2):106393. doi: 10.1016/j.ijantimicag.2021.106393. Epub 2021 Jun 24. Int J Antimicrob Agents. 2021. PMID: 34174409
References
-
- Finch N., Zasowski E., Murray K., Mynatt R., Zhao J., Yost R., et al. . (2017). A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob. Agents Chemother. 61 (12), e01293-17. doi: 10.1128/aac.01293-17 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

