Evaluation of Mycobacterium avium subsp. paratuberculosis isocitrate lyase (IcL) and ABC transporter (BacA) knockout mutants as vaccine candidates
- PMID: 37065210
- PMCID: PMC10098363
- DOI: 10.3389/fcimb.2023.1149419
Evaluation of Mycobacterium avium subsp. paratuberculosis isocitrate lyase (IcL) and ABC transporter (BacA) knockout mutants as vaccine candidates
Abstract
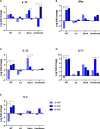
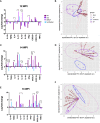
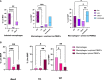
There has been little success in controlling Johne's disease, caused by Mycobacterium avium subsp. paratuberculosis, due to suboptimal diagnostics and the ineffectiveness of available vaccines. By knocking out BacA and IcL, genes required for MAP survival in dairy calves, two live-attenuated vaccine candidates were created. This study evaluated the host-specific attenuation of MAP IcL and BacA mutants in mouse and calf models, as well as the elicited immune responses. Deletion mutants were generated in MAP strain A1-157 through specialized transduction and found viable in vitro. First, the mutants' attenuation and elicited cytokine secretion were assessed in a mouse model, 3 weeks after intraperitoneal inoculation with MAP strains. Later, vaccine strains were assessed in a natural host infection model where calves received 109CFU oral dose of MAP wild-type or mutant strains at 2 weeks old. Transcription levels of cytokines in PBMCs were evaluated at 12-, 14-, and 16-weeks post-inoculation (WPI) and MAP colonization in tissue was assessed at 4.5 months after inoculation. Whereas both vaccine candidates colonized mouse tissues similarly to wild-type strain, both failed to persist in calf tissues. In either mouse or calf models, gene deletion did not reduce immunogenicity. Instead, inoculation with ΔBacA induced a greater upregulation of proinflammatory cytokines than ΔIcL and wild-type in both models and a greater expansion of cytotoxic and memory T-cells than uninfected control in calves. ΔBacA and wild-type strains significantly increased secretion of IP-10, MIG, TNFα, and RANTES in mice serum compared to uninfected control. This agreed with upregulation of IL-12, IL-17, and TNFα in calves inoculated with ΔBacA at all time points. The ΔBacA also gave rise to greater populations of CD4+CD45RO+, and CD8+ cells than uninfected control calves at 16 WPI. Low survival rate of MAP in macrophages co-incubated with PBMCs isolated from the ΔBacA group indicated that these cell populations are capable of killing MAP. Overall, the immune response elicited by ΔBacA is stronger compared to ΔIcL and it is maintained over two different models and over time in calves. Further investigation is warranted to evaluate the BacA mutant's protection against MAP infection as a live attenuated vaccine candidate.
Keywords: Mycobacterium avium subsp. paratuberculosis; control programs; dairy calves; immune responses; live-attenuated vaccines; vaccine development.
Copyright © 2023 Eshraghisamani, Arrazuria, Luo and De Buck.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Immunogenicity and efficacy of an oral live-attenuated vaccine for bovine Johne's disease.Front Immunol. 2024 Jan 12;14:1307621. doi: 10.3389/fimmu.2023.1307621. eCollection 2023. Front Immunol. 2024. PMID: 38283338 Free PMC article.
-
Evaluation of two mutants of Mycobacterium avium subsp. paratuberculosis as candidates for a live attenuated vaccine for Johne's disease.Vaccine. 2011 Jun 24;29(29-30):4709-19. doi: 10.1016/j.vaccine.2011.04.090. Epub 2011 May 10. Vaccine. 2011. PMID: 21565243 Free PMC article.
-
Oral paratuberculosis vaccine efficacy and mucosal immunity in cattle.Vaccine. 2024 Dec 2;42(26):126447. doi: 10.1016/j.vaccine.2024.126447. Epub 2024 Oct 17. Vaccine. 2024. PMID: 39423453
-
A rational framework for evaluating the next generation of vaccines against Mycobacterium avium subspecies paratuberculosis.Front Cell Infect Microbiol. 2014 Sep 9;4:126. doi: 10.3389/fcimb.2014.00126. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25250245 Free PMC article. Review.
-
Susceptibility to and diagnosis of Mycobacterium avium subspecies paratuberculosis infection in dairy calves: A review.Prev Vet Med. 2015 Oct 1;121(3-4):189-98. doi: 10.1016/j.prevetmed.2015.08.011. Epub 2015 Aug 20. Prev Vet Med. 2015. PMID: 26321657 Review.
Cited by
-
Modeling Paratuberculosis in Laboratory Animals, Cells, or Tissues: A Focus on Their Applications for Pathogenesis, Diagnosis, Vaccines, and Therapy Studies.Animals (Basel). 2023 Nov 17;13(22):3553. doi: 10.3390/ani13223553. Animals (Basel). 2023. PMID: 38003170 Free PMC article. Review.
-
A comprehensive review of paratuberculosis in animals and its implications for public health.Open Vet J. 2024 Nov;14(11):2731-2744. doi: 10.5455/OVJ.2024.v14.i11.2. Epub 2024 Nov 30. Open Vet J. 2024. PMID: 39737030 Free PMC article. Review.
-
Sheep challenged with sheep-derived type II Mycobacterium avium subsp. paratuberculosis: the first experimental model of paratuberculosis in China.BMC Vet Res. 2025 Apr 29;21(1):298. doi: 10.1186/s12917-025-04765-1. BMC Vet Res. 2025. PMID: 40301886 Free PMC article.
-
Immunogenicity and efficacy of an oral live-attenuated vaccine for bovine Johne's disease.Front Immunol. 2024 Jan 12;14:1307621. doi: 10.3389/fimmu.2023.1307621. eCollection 2023. Front Immunol. 2024. PMID: 38283338 Free PMC article.
-
Mycobacterium avium subsp. paratuberculosis Candidate Vaccine Strains Are Pro-apoptotic in RAW 264.7 Murine Macrophages.Vaccines (Basel). 2023 Jun 10;11(6):1085. doi: 10.3390/vaccines11061085. Vaccines (Basel). 2023. PMID: 37376474 Free PMC article.
References
-
- Abdellrazeq G. S., Elnaggar M. M., Bannantine J. P., Park K. T., Souza C. D., Backer B., et al. . (2018). A mycobacterium avium subsp. paratuberculosis relA deletion mutant and a 35 kDa major membrane protein elicit development of cytotoxic T lymphocytes with ability to kill intracellular bacteria. Vet. Res. 49, 1–16. doi: 10.1186/s13567-018-0549-3 - DOI - PMC - PubMed
-
- Ahlstrom C., Barkema H. W., Stevenson K., Zadoks R. N., Biek R., Kao R., et al. . (2015). Limitations of variable number of tandem repeat typing identified through whole genome sequencing of mycobacterium avium subsp. paratuberculosis on a national and herd level. BMC Genomics 16, 1–9. doi: 10.1186/s12864-015-1387-6 - DOI - PMC - PubMed
-
- Alonso-Hearn M., Molina E., Geijo M., Vazquez P., Sevilla I. A., Garrido J. M., et al. . (2012). Immunization of adult dairy cattle with a new heat-killed vaccine is associated with longer productive life prior to cows being sent to slaughter with suspected paratuberculosis. J. Dairy Sci. 95, 618–629. doi: 10.3168/jds.2009-2860 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

