Melatonin ameliorates serobiochemical alterations and restores the cardio-nephro diabetic vascular and cellular alterations in streptozotocin-induced diabetic rats
- PMID: 37065258
- PMCID: PMC10102477
- DOI: 10.3389/fvets.2023.1089733
Melatonin ameliorates serobiochemical alterations and restores the cardio-nephro diabetic vascular and cellular alterations in streptozotocin-induced diabetic rats
Erratum in
-
Corrigendum: Melatonin ameliorates serobiochemical alterations and restores the cardio-nephro diabetic vascular and cellular alterations in streptozotocin-induced diabetic rats.Front Vet Sci. 2023 Jul 18;10:1240320. doi: 10.3389/fvets.2023.1240320. eCollection 2023. Front Vet Sci. 2023. PMID: 37533456 Free PMC article.
Abstract
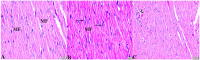
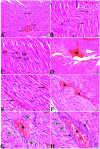
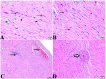
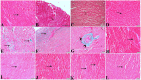
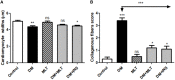
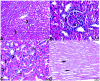
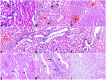
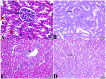
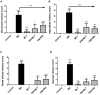
Melatonin possesses a wide range of pharmacological activities, including antidiabetic properties. Diabetes mellitus (DM) induces several physiopathological changes in body organs, which could be observed lately after systemic failure. In the current study, we aimed to investigate the serobiochemical changes and the histopathological picture in the diabetic heart and the kidney early before chronic complications and highlight the association between hyperglycemia, glomerular alterations, and cardiovascular changes. In addition, the role of melatonin in the treatment of cardio-nephro diabetic vascular and cellular adverse changes in streptozotocin-induced diabetic rats was also studied. A total of 40 mature Wistar albino rats were distributed into five groups; (1) control untreated rats, (2) diabetic mellitus untreated (DM) rats, in which DM was induced by the injection of streptozotocin (STZ), (3) control melatonin-treated (MLT), (4) melatonin-treated diabetic (DM + MLT) rats, in which melatonin was injected (10 mg/kg/day, i.p.) for 4 weeks, and (5) insulin-treated diabetic (DM + INS) rats. The serum biochemical analysis of diabetic STZ rats showed a significant (P < 0.05) increase in the concentrations of blood glucose, total oxidative capacity (TOC), CK-MB, endothelin-1, myoglobin, H-FABP, ALT, AST, urea, and creatinine as compared to control rats. In contrast, there was a significant (P < 0.05) decrease in serum concentration of insulin, total antioxidative capacity (TAC), total nitric oxide (TNO), and total protein level in DM rats vs. the control rats. Significant improvement in the serobiochemical parameters was noticed in both (DM + MLT) and (DM + INS) groups as compared with (DM) rats. The histological examination of the DM group revealed a disorder of myofibers, cardiomyocyte nuclei, and an increase in connective tissue deposits in between cardiac tissues. Severe congestion and dilation of blood capillaries between cardiac muscle fibers were also observed. The nephropathic changes in DM rats revealed various deteriorations in glomeruli and renal tubular cells of the same group. In addition, vascular alterations in the arcuate artery at the corticomedullary junction and interstitial congestion take place. Melatonin administration repaired all these histopathological alterations to near-control levels. The study concluded that melatonin could be an effective therapeutic molecule for restoring serobiochemical and tissue histopathological alterations during diabetes mellitus.
Keywords: H-FABP; TNO; endothelin-1; heart; kidney; melatonin.
Copyright © 2023 Elmahallawy, Alsharif, Alblihd, Hamad, Nasreldin, Alsanie, Aljoudi, Oyouni, Al-Amer, Albarakati, Lokman, Albrakati and Ali.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Adam M, Tan JH, Ng EY. The effect of diabetes on cardiovascular system. J Mech Med Biol. (2017) 17:1740008. 10.1142/S0219519417400085 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

