Gene Therapy for Spinal Muscular Atrophy (SMA): A Review of Current Challenges and Safety Considerations for Onasemnogene Abeparvovec (Zolgensma)
- PMID: 37065340
- PMCID: PMC10104684
- DOI: 10.7759/cureus.36197
Gene Therapy for Spinal Muscular Atrophy (SMA): A Review of Current Challenges and Safety Considerations for Onasemnogene Abeparvovec (Zolgensma)
Abstract
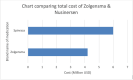
Spinal Muscular Atrophy (SMA) is a genetic disease that causes weakness and wasting in the voluntary muscles of infants and children. SMA has been the leading inherited cause of infant death. More specifically, SMA is caused by the absence of the SMN1 gene. In May 2019, the Food and Drug Administration (FDA) approved onasemnogene abeparvovec, SMN1 gene replacement therapy, for all children with SMA younger than two years of age, without end-stage weakness. The objective of the study is to review the safety and efficacy of a novel gene therapy, onasemnogene abeparvovec (Zolgensma), for SMA and assess current challenges for gene therapy. For this, we have conducted a literature search on PubMed, MEDLINE, and Ovid (2019 to 2022) in the English language using the terms SMA, onasemnogene, and gene therapy. The search included articles, websites, and published papers from reputable health organizations, hospitals, and global organizations dedicated to bringing awareness to Spinal Muscular Atrophy. We found the first gene therapy for SMA to be onasemnogene, directly providing the survival motor neuron 1 (SMN1) gene to produce the survival motor neuron (SMN) protein. Onasemnogene is approved by the Food and Drug Administration and has the added benefit of being a one-time dose. On the downside, a major side effect of this treatment is hepatotoxicity. There is substantial evidence that the efficacy of therapy is increased when administered early to children under three months of age. Therefore, we concluded that onasemnogene appears to be an efficacious therapy for younger pediatric patients with SMA type 1. Drug cost and potential hepatotoxicity are major concerns. Long-term benefits and risks have not been determined, but it is more cost-effective and requires less time of treatment compared to the other used drug, nusinersen. Therefore, the combined safety, cost, and effectiveness of onasemnogene abeparvovec make it a reliable treatment option for treating SMA Type 1.
Keywords: avxs-101; gene therapy; gene-replacement therapy; onasemnogene abeparvovec; sma; sma 1; sma 2; spinal muscular atrophy; spinal muscular atrophy treatment for infant; zolgensma.
Copyright © 2023, Ogbonmide et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Survival, motor function, and motor milestones: comparison of AVXS-101 relative to nusinersen for the treatment of infants with spinal muscular atrophy type 1. Dabbous O, Maru B, Jansen JP, et al. https://doi.org/10.1007/s12325-019-00923-8. Adv Ther. 2019;36:1164–1176. - PMC - PubMed
-
- Spinal muscular atrophy: past, present, and future. Ross LF, Kwon JM. https://doi.org/10.1542/neo.20-8-e437 Neoreviews. 2019;20:0–51. - PubMed
-
- Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. Chand D, Mohr F, McMillan H, et al. https://doi.org/10.1016/j.jhep.2020.11.001. J Hepatol. 2021;74:560–566. - PubMed
-
- Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Lowes LP, Alfano LN, Arnold WD, et al. https://doi.org/10.1016/j.pediatrneurol.2019.05.005. Pediatr Neurol. 2019;98:39–45. - PubMed
Publication types
LinkOut - more resources
Full Text Sources