Dynamics of oral microbiome acquisition in healthy infants: A pilot study
- PMID: 37065420
- PMCID: PMC10098328
- DOI: 10.3389/froh.2023.1152601
Dynamics of oral microbiome acquisition in healthy infants: A pilot study
Abstract
Objectives: The human oral microbiota is one of the most complex bacterial communities in the human body. However, how newborns initially acquire these bacteria remains largely unknown. In this study, we examined the dynamics of oral microbial communities in healthy infants and investigated the influence of the maternal oral microbiota on the acquisition of the infant's oral microbiota. We hypothesized that the infant oral microbial diversity increases with age.
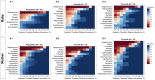
Methods: One hundred and sixteen whole-salivary samples were collected from 32 healthy infants and their biological mothers during postpartum and 9- and 15-month well-infant visits. Bacterial genomic DNA was extracted and sequenced by Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) methods. The Shannon index was used to measure the microbial diversity of the infant-mother dyads (alpha diversity). The microbial diversity between the mother-infant dyads (beta-diversity) was calculated using the weighted non-phylogenetic Bray-Curtis distance in QIIME 1.9.1. Core microbiome analysis was performed using MicrobiomeAnalyst software. Linear discriminant analysis coupled with effect size analysis was used to identify differentially abundant features between mother and infant dyads.
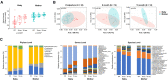
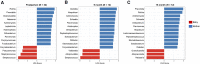
Results: A total of 6,870,571 16S rRNA reads were generated from paired mother-infant saliva samples. Overall, oral microbial profiles significantly differed between the mother and infant groups (p < 0.001). The diversity of the salivary microbiomes in the infants increased in an age-dependent manner, whereas the core microbiome of the mothers remained relatively stable during the study period. Breastfeeding and gender did not affect the microbial diversity in infants. Moreover, infants had a greater relative abundance of Firmicutes and a lower abundance of Actinobacteria, Bacteroidetes, Fusobacteria, and Proteobacteria than their mothers. The SparCC correlation analysis demonstrated constant changes in infants' oral microbial community network (p < 0.05).
Conclusions: This study provides new evidence that the oral cavities of infants are colonized by a distinct group of bacterial species at birth. The acquisition and diversity of changes in oral microbial composition are dynamic during the first year of an infant's life. Before reaching the second birthday, the composition of the oral microbial community could be more similar to that of their biological mothers.
Keywords: infant-mother dyads; microbial diversity; microbial initial acquisition; oral microbiota; postpartum.
© 2023 Li, Saraithong, Zhang, Dills, Paster, Xiao, Wu and Jones.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Strong Multivariate Relations Exist Among Milk, Oral, and Fecal Microbiomes in Mother-Infant Dyads During the First Six Months Postpartum.J Nutr. 2019 Jun 1;149(6):902-914. doi: 10.1093/jn/nxy299. J Nutr. 2019. PMID: 31063198 Free PMC article.
-
Salivary microbiomes of indigenous Tsimane mothers and infants are distinct despite frequent premastication.PeerJ. 2016 Nov 3;4:e2660. doi: 10.7717/peerj.2660. eCollection 2016. PeerJ. 2016. PMID: 27833819 Free PMC article.
-
Breastfeeding patterns are associated with human milk microbiome composition: The Mother-Infant Microbiomes, Behavior, and Ecology Study (MIMBES).PLoS One. 2023 Aug 9;18(8):e0287839. doi: 10.1371/journal.pone.0287839. eCollection 2023. PLoS One. 2023. PMID: 37556398 Free PMC article.
-
Postpartum Maternal Stress is Unrelated to the Infant Fecal Microbiome, but is Associated With the Human Milk Microbiome in Exclusively Breastfeeding Mother-Infant Dyads: The Mother-Infant Microbiomes, Behavior, and Ecology Study (MIMBES).Am J Hum Biol. 2025 May;37(5):e70061. doi: 10.1002/ajhb.70061. Am J Hum Biol. 2025. PMID: 40387412 Free PMC article.
-
Getting ahead of human-associated microbial decline in Africa: the urgency of sampling in light of epidemiological transition.Trends Microbiol. 2025 Feb 27:S0966-842X(25)00005-8. doi: 10.1016/j.tim.2025.01.004. Online ahead of print. Trends Microbiol. 2025. PMID: 40021386 Review.
Cited by
-
Oral Microbiota: The Influences and Interactions of Saliva, IgA, and Dietary Factors in Health and Disease.Microorganisms. 2023 Sep 13;11(9):2307. doi: 10.3390/microorganisms11092307. Microorganisms. 2023. PMID: 37764151 Free PMC article. Review.
-
Impact of breastfeeding and other early-life factors on the development of the oral microbiome.Front Microbiol. 2023 Sep 7;14:1236601. doi: 10.3389/fmicb.2023.1236601. eCollection 2023. Front Microbiol. 2023. PMID: 37744908 Free PMC article. Review.
-
Development of the breastfed infant oral microbiome over the first two years of life in the BLOSOM Cohort.Front Cell Infect Microbiol. 2025 Apr 15;15:1534750. doi: 10.3389/fcimb.2025.1534750. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40302925 Free PMC article.
-
A Comparative Analysis of Feeding Practices and Oral Immunity in Infants.Medicina (Kaunas). 2025 Jun 19;61(6):1114. doi: 10.3390/medicina61061114. Medicina (Kaunas). 2025. PMID: 40572802 Free PMC article.
-
Episymbiotic Saccharibacteria suppresses epithelial immunoactivation through Type IV pili and TLR2 dependent endocytosis.bioRxiv [Preprint]. 2025 Jun 2:2025.05.30.656655. doi: 10.1101/2025.05.30.656655. bioRxiv. 2025. PMID: 40501963 Free PMC article. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

