Efficacy and safety of immune checkpoint inhibitors versus chemotherapy in the second-line treatment of advanced esophageal squamous cell carcinoma: a meta-analysis and systematic review
- PMID: 37065572
- PMCID: PMC10089833
- DOI: 10.21037/jtd-22-1169
Efficacy and safety of immune checkpoint inhibitors versus chemotherapy in the second-line treatment of advanced esophageal squamous cell carcinoma: a meta-analysis and systematic review
Abstract
Background: Esophageal cancer (EC) is the seventh most common cancer in the world, with 604,000 new cases diagnosed each year. Immune checkpoint inhibitors (ICIs) including programmed death ligand-1 (PD-L1) inhibitors have demonstrated a considerable survival advantage over chemotherapy in numerous randomized controlled trials (RCTs), particularly in patients with advanced esophageal squamous cell carcinoma (ESCC). In this analysis, we aimed to demonstrate that ICIs are more safe and effective than chemotherapy when used as a second-line treatment for advanced ESCC.
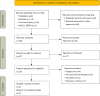
Methods: Publications on the safety and efficiency of ICIs in advanced ESCC that were available prior to February 2022 were searched in the Cochrane Library, Embase, and PubMed databases. Studies with missing data were eliminated, and studies that compared the treatments between the immunotherapy group and chemotherapy group were included. Statistical analysis was carried out using RevMan 5.3, and risk and quality were evaluated with relevant evaluation tools.
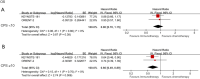
Results: Five studies met the inclusion criteria were selected, involving 1,970 patients with advanced ESCC. We compared chemotherapy and immunotherapy in the second-line treatment of advanced ESCC. ICIs considerably enhanced both the objective response rate (P=0.007) and overall survival (OS; P=0.001). However, the effect of ICIs on progression-free survival (PFS) was not significant (P=0.43). ICIs presented fewer grade 3-5 treatment-related adverse events (TRAEs), and there was also a suggested linkage between both PD-L1 expression and the effectiveness of the therapeutic intervention.
Conclusions: For patients with advanced ESCC, ICIs are more effective and safer than chemotherapy, and thus have a higher treatment value.
Keywords: Esophageal squamous cell carcinoma (ESCC); immune checkpoint inhibitors (ICIs); meta-analysis.
2023 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1169/coif). The authors have no conflicts of interest to declare.
Figures



Comment in
-
Immune checkpoint inhibition in advanced esophageal squamous cell carcinoma: how can we personalise management?J Thorac Dis. 2023 Jul 31;15(7):3525-3528. doi: 10.21037/jtd-23-598. Epub 2023 Jun 20. J Thorac Dis. 2023. PMID: 37559602 Free PMC article. No abstract available.
Similar articles
-
Clinical benefits of PD-1 inhibitors in specific subgroups of patients with advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis of phase 3 randomized clinical trials.Front Immunol. 2023 May 2;14:1171671. doi: 10.3389/fimmu.2023.1171671. eCollection 2023. Front Immunol. 2023. PMID: 37205107 Free PMC article.
-
PD-1 inhibitors versus chemotherapy as second-line treatment for advanced esophageal squamous cell carcinoma: a meta-analysis.BMC Cancer. 2021 Nov 10;21(1):1195. doi: 10.1186/s12885-021-08958-3. BMC Cancer. 2021. PMID: 34758782 Free PMC article.
-
Clinical efficacy of combination therapy of an immune checkpoint inhibitor with taxane plus platinum versus an immune checkpoint inhibitor with fluorouracil plus platinum in the first-line treatment of patients with locally advanced, metastatic, or recurrent esophageal squamous cell carcinoma.Front Oncol. 2022 Dec 20;12:1015302. doi: 10.3389/fonc.2022.1015302. eCollection 2022. Front Oncol. 2022. PMID: 36605427 Free PMC article.
-
Comparison of neoadjuvant immunotherapy plus chemotherapy versus chemotherapy alone for patients with locally advanced esophageal squamous cell carcinoma: A propensity score matching.Front Immunol. 2022 Oct 7;13:970534. doi: 10.3389/fimmu.2022.970534. eCollection 2022. Front Immunol. 2022. PMID: 36275724 Free PMC article.
-
PD-1/PD-L1 immune checkpoint inhibitors in metastatic triple-negative breast cancer: a systematic review and meta-analysis.Front Immunol. 2023 Jun 12;14:1206689. doi: 10.3389/fimmu.2023.1206689. eCollection 2023. Front Immunol. 2023. PMID: 37377959 Free PMC article.
Cited by
-
Advancements in the research of immune checkpoint inhibitors for the treatment of advanced esophageal squamous cell carcinoma.Am J Cancer Res. 2024 May 15;14(5):1981-1998. doi: 10.62347/XUWC6412. eCollection 2024. Am J Cancer Res. 2024. PMID: 38859835 Free PMC article. Review.
-
Efficacy and safety of PD-1 inhibitors as second-line treatment for advanced squamous esophageal cancer: a systematic review and network meta-analysis with a focus on PD-L1 expression levels.Front Immunol. 2025 Jan 23;15:1510145. doi: 10.3389/fimmu.2024.1510145. eCollection 2024. Front Immunol. 2025. PMID: 39916953 Free PMC article.
-
Immune checkpoint inhibition in advanced esophageal squamous cell carcinoma: how can we personalise management?J Thorac Dis. 2023 Jul 31;15(7):3525-3528. doi: 10.21037/jtd-23-598. Epub 2023 Jun 20. J Thorac Dis. 2023. PMID: 37559602 Free PMC article. No abstract available.
-
Breaking Barriers: The Promise and Challenges of Immune Checkpoint Inhibitors in Triple-Negative Breast Cancer.Biomedicines. 2024 Feb 5;12(2):369. doi: 10.3390/biomedicines12020369. Biomedicines. 2024. PMID: 38397971 Free PMC article. Review.
-
Utilising systematic reviews to assess potential overtreatment and claim for better evidence-based research: an analysis of anticancer drugs versus supportive care in advanced esophageal cancer.Syst Rev. 2024 Jul 18;13(1):186. doi: 10.1186/s13643-024-02594-1. Syst Rev. 2024. PMID: 39026378 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
