Perioperative outcomes of neoadjuvant immunotherapy plus chemotherapy and neoadjuvant chemoradiotherapy in the treatment of locally advanced esophageal squamous cell carcinoma: a retrospective comparative cohort study
- PMID: 37065590
- PMCID: PMC10089853
- DOI: 10.21037/jtd-23-84
Perioperative outcomes of neoadjuvant immunotherapy plus chemotherapy and neoadjuvant chemoradiotherapy in the treatment of locally advanced esophageal squamous cell carcinoma: a retrospective comparative cohort study
Abstract
Background: Neoadjuvant chemoradiotherapy (nCRT) is recommended as the preferred treatment for locally advanced esophageal squamous cell carcinoma. Recent studies have shown that immune checkpoint inhibitors are beneficial in treating advanced esophageal cancer. Therefore, a growing number of clinical centers are conducting trials of neoadjuvant immunotherapy or neoadjuvant immunotherapy plus chemotherapy (nICT) in patients with locally advanced resectable esophageal cancer. Immunocheckpoint inhibitors are expected to play a role in neoadjuvant therapy for esophageal cancer. However, there were few studies comparing nICT with nCRT. This study compared the efficacy and safety of nICT with that of nCRT administered prior to esophagectomy in patients with resectable locally advanced esophageal squamous cell carcinoma (ESCC).
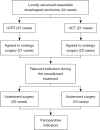
Methods: The study included patients with locally advanced resectable ESCC who were scheduled to receive neoadjuvant therapy at Gaozhou People's Hospital from January 1, 2019, to September 1, 2022. The enrolled patients were divided into 2 groups (nCRT or nICT) according to their neoadjuvant therapy regimen. The 2 groups were compared for their baseline data, the incidence of adverse events during neoadjuvant therapy, the clinical evaluation after neoadjuvant therapy, perioperative indicators, and the incidence of postoperative complications and postoperative pathological remission.
Results: A total of 44 patients were enrolled; 23 in the nCRT group and 21 in the nICT group. There were no significant differences between the 2 groups in the baseline data. In the nCRT group, leukopenia occurred more often than in the nICT group, and hemoglobin-decreasing events were rarer (P=0.03<0.05). A significantly higher proportion of patients in the nICT group experienced erythema following neoadjuvant therapy compared to the nCRT group (23.81% vs. 0%; P=0.01<0.05). Neoadjuvant therapy showed no significant difference between the 2 groups for adverse event rates, surgery-related indicators, postoperative pathological remission rates, and postoperative complications.
Conclusions: nICT was a safe and feasible treatment for locally advanced ESCC and it may be a potential new treatment modality.
Keywords: Esophageal cancer; chemoradiotherapy; chemotherapy; immunotherapy; neoadjuvant.
2023 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-84/coif). The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Pathologic responses and surgical outcomes after neoadjuvant immunochemotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma.Front Immunol. 2022 Nov 17;13:1052542. doi: 10.3389/fimmu.2022.1052542. eCollection 2022. Front Immunol. 2022. PMID: 36466925 Free PMC article.
-
Comparison of pathologic response and survival outcomes between neoadjuvant chemoradiotherapy (nCRT) and neoadjuvant immunochemotherapy (nICT) in patients with locally advanced esophageal squamous cell carcinoma: a propensity score-matched analysis.BMC Cancer. 2024 Oct 5;24(1):1228. doi: 10.1186/s12885-024-12946-8. BMC Cancer. 2024. PMID: 39369225 Free PMC article.
-
Comparison of neoadjuvant chemotherapy plus immunotherapy versus chemoradiotherapy for esophageal squamous cell carcinoma patients: efficacy and safety outcomes.J Thorac Dis. 2025 May 30;17(5):2937-2946. doi: 10.21037/jtd-2024-2107. Epub 2025 May 23. J Thorac Dis. 2025. PMID: 40529751 Free PMC article.
-
Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: A systematic review and meta-analysis.Front Immunol. 2023 Mar 23;14:1108213. doi: 10.3389/fimmu.2023.1108213. eCollection 2023. Front Immunol. 2023. PMID: 37033991 Free PMC article.
-
Neoadjuvant immune checkpoint inhibitor in combination with chemotherapy or chemoradiotherapy in resectable esophageal cancer: A systematic review and meta-analysis.Front Immunol. 2022 Sep 13;13:998620. doi: 10.3389/fimmu.2022.998620. eCollection 2022. Front Immunol. 2022. PMID: 36177019 Free PMC article.
Cited by
-
Neoadjuvant arterial infusion chemotherapy combined with immunotherapy in treating locally advanced lower esophageal and esophagogastric junction cancer.J Thorac Dis. 2025 Apr 30;17(4):2273-2285. doi: 10.21037/jtd-24-1908. Epub 2025 Apr 23. J Thorac Dis. 2025. PMID: 40400978 Free PMC article.
References
-
- Yu Y, Xu L, Chen X, et al. Neoadjuvant therapy combined with surgery is superior to chemoradiotherapy in esophageal squamous cell cancer patients with resectable supraclavicular lymph node metastasis: a propensity score-matched analysis. Ann Transl Med 2022;10:349. 10.21037/atm-22-577 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
