Degradation-Resistant Hypoxia Inducible Factor-2α in Murine Osteocytes Promotes a High Bone Mass Phenotype
- PMID: 37065633
- PMCID: PMC10097640
- DOI: 10.1002/jbm4.10724
Degradation-Resistant Hypoxia Inducible Factor-2α in Murine Osteocytes Promotes a High Bone Mass Phenotype
Abstract
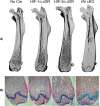
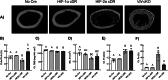
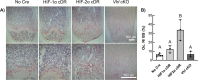
Molecular oxygen levels vary during development and disease. Adaptations to decreased oxygen bioavailability (hypoxia) are mediated by hypoxia-inducible factor (HIF) transcription factors. HIFs are composed of an oxygen-dependent α subunit (HIF-α), of which there are two transcriptionally active isoforms (HIF-1α and HIF-2α), and a constitutively expressed β subunit (HIFβ). Under normoxic conditions, HIF-α is hydroxylated via prolyl hydroxylase domain (PHD) proteins and targeted for degradation via Von Hippel-Lindau (VHL). Under hypoxic conditions, hydroxylation via PHD is inhibited, allowing for HIF-α stabilization and induction of target transcriptional changes. Our previous studies showed that Vhl deletion in osteocytes (Dmp1-cre; Vhl f/f ) resulted in HIF-α stabilization and generation of a high bone mass (HBM) phenotype. The skeletal impact of HIF-1α accumulation has been well characterized; however, the unique skeletal impacts of HIF-2α remain understudied. Because osteocytes orchestrate skeletal development and homeostasis, we investigated the role of osteocytic HIF-α isoforms in driving HBM phenotypes via osteocyte-specific loss-of-function and gain-of-function HIF-1α and HIF-2α mutations in C57BL/6 female mice. Deletion of Hif1a or Hif2a in osteocytes showed no effect on skeletal microarchitecture. Constitutively stable, degradation-resistant HIF-2α (HIF-2α cDR), but not HIF-1α cDR, generated dramatic increases in bone mass, enhanced osteoclast activity, and expansion of metaphyseal marrow stromal tissue at the expense of hematopoietic tissue. Our studies reveal a novel influence of osteocytic HIF-2α in driving HBM phenotypes that can potentially be harnessed pharmacologically to improve bone mass and reduce fracture risk. © 2023 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: GENETIC ANIMAL MODELS; OSTEOCLAST; OSTEOCYTE.
© 2023 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Conflict of interest statement
All authors declare that they have no relevant or material financial interests that relate to the research described in this paper.
Figures



References
-
- Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393‐402. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
