Hybrid biofabrication of neurosecretory structures as a model for neurosecretion
- PMID: 37065654
- PMCID: PMC10090530
- DOI: 10.18063/ijb.v9i2.659
Hybrid biofabrication of neurosecretory structures as a model for neurosecretion
Abstract
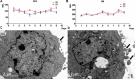
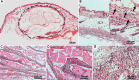
The present study aimed to combine extrusion-based three-dimensional (3D) bioprinting and polymer nanofiber electrospinning technology to fabricate tissue-like structures with neurosecretory function in vitro. Using neurosecretory cells as cell resources, sodium alginate/gelatin/fibrinogen as matrix, polylactic acid/gelatin electrospun nanofibers as diaphragm, and neurosecretory cells-loaded 3D hydrogel scaffolds were bioprinted and then covered with electrospun nanofibers layer-by-layer. The morphology was observed by scanning electron microscopy and transmission electron microscopy (TEM), and the mechanical characteristics and cytotoxicity of the hybrid biofabricated scaffold structure were evaluated. The 3D-bioprinted tissue activity, including cell death and proliferation, was verified. Western blotting and ELISA experiments were used to confirm the cell phenotype and secretory function, while animal in vivo transplantation experiments confirmed the histocompatibility, inflammatory reaction, and tissue remodeling ability of the heterozygous tissue structures. Neurosecretory structures with 3D structures were successfully prepared by hybrid biofabrication in vitro. The mechanical strength of the composite biofabricated structures was significantly higher than that of the hydrogel system (P < 0.05). The survival rate of PC12 cells in the 3D-bioprinted model was 92.849 ± 2.995%. Hematoxylin and eosin-stained pathological sections showed that the cells grew in clumps, and there was no significant difference in the expression of MAP2 and tubulin-β between 3D organoids and PC12 cells. The results of ELISA showed that the PC12 cells in 3D structures retained the ability to continuously secrete noradrenaline and met-enkephalin, and the secretory vesicles around and within the cells could be observed by TEM. In in vivo transplantation, PC12 cells gathered and grew in clusters, maintained high activity, neovascularization, and tissue remodeling in 3D structures. The neurosecretory structures were biofabricated by 3D bioprinting and nanofiber electrospinning in vitro, which had high activity and neurosecretory function. In vivo transplantation of neurosecretory structures showed active proliferation of cells and potential for tissue remodeling. Our research provides a new method for biological manufacture of neurosecretory structures in vitro, which maintains neurosecretory function and lays the foundation for the clinical application of neuroendocrine tissues.
Keywords: Bioprinting; Electrospinning; Hybrid biofabrication; Neurosecretion; Structures.
Copyright: © 2022 Author(s).
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
ECM concentration and cell-mediated traction forces play a role in vascular network assembly in 3D bioprinted tissue.Biotechnol Bioeng. 2020 Apr;117(4):1148-1158. doi: 10.1002/bit.27250. Epub 2020 Jan 11. Biotechnol Bioeng. 2020. PMID: 31840798
-
Wet electrospun alginate/gelatin hydrogel nanofibers for 3D cell culture.Int J Biol Macromol. 2018 Oct 15;118(Pt B):1648-1654. doi: 10.1016/j.ijbiomac.2018.07.005. Epub 2018 Jul 4. Int J Biol Macromol. 2018. PMID: 29981331
-
Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering.Acta Biomater. 2020 Sep 15;114:307-322. doi: 10.1016/j.actbio.2020.07.016. Epub 2020 Jul 13. Acta Biomater. 2020. PMID: 32673752
-
3D bioprinting and the revolution in experimental cancer model systems-A review of developing new models and experiences with in vitro 3D bioprinted breast cancer tissue-mimetic structures.Pathol Oncol Res. 2023 Feb 9;29:1610996. doi: 10.3389/pore.2023.1610996. eCollection 2023. Pathol Oncol Res. 2023. PMID: 36843955 Free PMC article. Review.
-
Extrusion 3D (Bio)Printing of Alginate-Gelatin-Based Composite Scaffolds for Skeletal Muscle Tissue Engineering.Materials (Basel). 2022 Nov 10;15(22):7945. doi: 10.3390/ma15227945. Materials (Basel). 2022. PMID: 36431432 Free PMC article. Review.
Cited by
-
Gene expression profiling and the isocitrate dehydrogenase mutational landscape of temozolomide‑resistant glioblastoma.Oncol Lett. 2024 Jun 17;28(2):378. doi: 10.3892/ol.2024.14511. eCollection 2024 Aug. Oncol Lett. 2024. PMID: 38939621 Free PMC article.
-
ITGB2 fosters the cancerous characteristics of ovarian cancer cells through its role in mitochondrial glycolysis transformation.Aging (Albany NY). 2024 Feb 11;16(3):3007-3020. doi: 10.18632/aging.205529. Epub 2024 Feb 11. Aging (Albany NY). 2024. PMID: 38345576 Free PMC article.
References
-
- Yoo ES, Yu J, Sohn JW. Neuroendocrine control of appetite and metabolism. Exp Mol Med. 2021;53:505–516. https://doi.org/10.1038/s12276-021-00597-9. - PMC - PubMed
-
- Téblick A, Gunst J, Langouche L, et al. Novel insights in endocrine and metabolic pathways in sepsis and gaps for future research. Clin Sci (Lond) 2022;136:861–878. https://doi.org/10.1042/CS20211003. - PubMed
-
- Garrahy A, Sherlock M, Thompson CJ. Management of endocrine disease:Neuroendocrine surveillance and management of neurosurgical patients. Eur J Endocrinol. 2017;176:R217–R233. https://doi.org/10.1530/EJE-16-0962. - PubMed
-
- Schwerdtfeger LA, Tobet SA. From organotypic culture to body-on-a-chip:A neuroendocrine perspective. J Neuroendocrinol. 2019;31:e12650. https://doi.org/10.1111/jne.12650. - PubMed
-
- Jo Y, Hwang DG, Kim M, et al. Bioprinting-assisted tissue assembly to generate organ substitutes at scale. Trends Biotechnol. 2022 S0167-7799(22)00170-6. https://doi.org/10.1016/j.tibtech.2022.07.001. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
