316Engineered extracellular vesicle-mediated delivery of miR-199a-3p increases the viability of 3D-printed cardiac patches
- PMID: 37065655
- PMCID: PMC10090809
- DOI: 10.18063/ijb.v9i2.670
316Engineered extracellular vesicle-mediated delivery of miR-199a-3p increases the viability of 3D-printed cardiac patches
Abstract
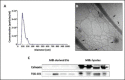
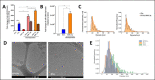
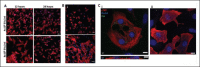
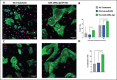
In recent years, extrusion-based three-dimensional (3D) bioprinting is employed for engineering cardiac patches (CP) due to its ability to assemble complex structures from hydrogel-based bioinks. However, the cell viability in such CPs is low due to shear forces applied on the cells in the bioink, inducing cellular apoptosis. Herein, we investigated whether the incorporation of extracellular vesicles (EVs) in the bioink, engineered to continually deliver the cell survival factor miR-199a-3p would increase the viability within the CP. EVs from THP-1-derived activated macrophages (MΦ) were isolated and characterized by nanoparticle tracking analysis (NTA), cryogenic electron microscopy (cryo-TEM), and Western blot analysis. MiR-199a-3p mimic was loaded into EVs by electroporation after optimization of applied voltage and pulses. Functionality of the engineered EVs was assessed in neonatal rat cardiomyocyte (NRCM) monolayers using immunostaining for the proliferation markers ki67 and Aurora B kinase. To examine the effect of engineered EVs on 3D-bioprinted CP viability, the EVs were added to the bioink, consisting of alginate-RGD, gelatin, and NRCM. Metabolic activity and expression levels of activated-caspase 3 for apoptosis of the 3D-bioprinted CP were evaluated after 5 days. Electroporation (850 V with 5 pulses) was found to be optimal for miR loading; miR-199a-3p levels in EVs increased fivefold compared to simple incubation, with a loading efficiency of 21.0%. EV size and integrity were maintained under these conditions. Cellular uptake of engineered EVs by NRCM was validated, as 58% of cTnT+ cells internalized EVs after 24 h. The engineered EVs induced CM proliferation, increasing the ratio of cell-cycle re-entry of cTnT+ cells by 30% (Ki67) and midbodies+ cell ratio by twofold (Aurora B) compared with the controls. The inclusion of engineered EVs in bioink yielded CP with threefold greater cell viability compared to bioink with no EVs. The prolonged effect of EVs was evident as the CP exhibited elevated metabolic activities after 5 days, with less apoptotic cells compared to CP with no EVs. The addition of miR-199a-3p-loaded EVs to the bioink improved the viability of 3D-printed CP and is expected to contribute to their integration in vivo.
Keywords: 3D bioprinting; Cardiac patch; Cardiomyocytes; Extracellular vesicles; Tissue Engineering; miRNA.
Copyright: © 2023, Bar A, Kryukov O, Etzion S.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
Extracellular matrix-derived extracellular vesicles promote cardiomyocyte growth and electrical activity in engineered cardiac atria.Biomaterials. 2017 Nov;146:49-59. doi: 10.1016/j.biomaterials.2017.09.001. Epub 2017 Sep 4. Biomaterials. 2017. PMID: 28898757
-
Engineered hsa-miR-455-3p-Abundant Extracellular Vesicles Derived from 3D-Cultured Adipose Mesenchymal Stem Cells for Tissue-Engineering Hyaline Cartilage Regeneration.Adv Healthc Mater. 2024 Jul;13(18):e2304194. doi: 10.1002/adhm.202304194. Epub 2024 Apr 3. Adv Healthc Mater. 2024. PMID: 38508211
-
Anti-inflammatory, Anti-fibrotic and Pro-cardiomyogenic Effects of Genetically Engineered Extracellular Vesicles Enriched in miR-1 and miR-199a on Human Cardiac Fibroblasts.Stem Cell Rev Rep. 2023 Nov;19(8):2756-2773. doi: 10.1007/s12015-023-10621-2. Epub 2023 Sep 13. Stem Cell Rev Rep. 2023. PMID: 37700183 Free PMC article.
-
Bioprinting extracellular vesicles as a "cell-free" regenerative medicine approach.Extracell Vesicles Circ Nucl Acids. 2023 May 23;4(2):218-239. doi: 10.20517/evcna.2023.19. eCollection 2023. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 39697984 Free PMC article. Review.
-
A review on alginate-based bioinks, combination with other natural biomaterials and characteristics.J Biomater Appl. 2022 Aug;37(2):355-372. doi: 10.1177/08853282221085690. Epub 2022 May 5. J Biomater Appl. 2022. PMID: 35510845 Review.
Cited by
-
Current advances in the development of microRNA-integrated tissue engineering strategies: a cornerstone of regenerative medicine.Front Bioeng Biotechnol. 2024 Oct 16;12:1484151. doi: 10.3389/fbioe.2024.1484151. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39479296 Free PMC article. Review.
-
Hydrogel-mediated extracellular vesicles for enhanced wound healing: the latest progress, and their prospects for 3D bioprinting.J Nanobiotechnology. 2024 Feb 10;22(1):57. doi: 10.1186/s12951-024-02315-9. J Nanobiotechnology. 2024. PMID: 38341585 Free PMC article. Review.
-
Development of Covalently Functionalized Alginate-Pyrrole and Polypyrrole-Alginate Nanocomposites as 3D Printable Electroconductive Bioinks.Materials (Basel). 2025 Jul 1;18(13):3120. doi: 10.3390/ma18133120. Materials (Basel). 2025. PMID: 40649608 Free PMC article.
-
Exploring Synergistic Effects of Bioprinted Extracellular Vesicles for Skin Regeneration.Biomedicines. 2024 Jul 18;12(7):1605. doi: 10.3390/biomedicines12071605. Biomedicines. 2024. PMID: 39062178 Free PMC article. Review.
-
Hydrogels in Cardiac Surgery: Versatile Platforms for Tissue Repair, Adhesion Prevention, and Localized Therapeutics.Gels. 2025 Jul 21;11(7):564. doi: 10.3390/gels11070564. Gels. 2025. PMID: 40710725 Free PMC article. Review.
References
-
- Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol . 2005;23:845. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
