369Fabrication of 3D gel-printed β-tricalcium phosphate/titanium dioxide porous scaffolds for cancellous bone tissue engineering
- PMID: 37065658
- PMCID: PMC10090810
- DOI: 10.18063/ijb.v9i2.673
369Fabrication of 3D gel-printed β-tricalcium phosphate/titanium dioxide porous scaffolds for cancellous bone tissue engineering
Abstract
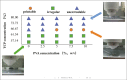
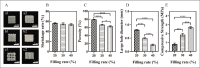
Human bone is composed of cortical bone and cancellous bone. The interior portion of natural bone is cancellous with a porosity of 50%-90%, but the outer layer is made of dense cortical bone, of which porosity was not higher than 10%. Porous ceramics were expected to be research hotspot in bone tissue engineering by virtue of their similarity to the mineral constituent and physiological structure of human bone. However, it is challenging to utilize conventional manufacturing methods to fabricate porous structures with precise shapes and pore sizes. Three-dimensional (3D) printing of ceramics is currently the latest research trend because it has many advantages in the fabrication of porous scaffolds, which can meet the requirements of cancellous bone strength, arbitrarily complex shapes, and individualized design. In this study, β-tricalcium phosphate (β-TCP)/titanium dioxide (TiO2) porous ceramics scaffolds were fabricated by 3D gel-printing sintering for the first time. The chemical constituent, microstructure, and mechanical properties of the 3D-printed scaffolds were characterized. After sintering, a uniform porous structure with appropriate porosity and pore sizes was observed. Besides, biological mineralization activity and biocompatibility were evaluated by in vitro cell assay. The results demonstrated that the incorporation of TiO2 (5 wt%) significantly improved the compressive strength of the scaffolds, with an increase of 283%. Additionally, the in vitro results showed that the β-TCP/TiO2 scaffold had no toxicity. Meanwhile, the adhesion and proliferation of MC3T3-E1 cells on scaffolds were desirable, revealing that the β-TCP/TiO2 scaffolds can be used as a promising candidate for repair scaffolding in orthopedics and traumatology.
Keywords: 3D printing; Bone tissue engineering; Porous scaffolds; Titanium dioxide; β-tricalcium phosphate.
Copyright: © 2023, Hu X, Li H, Qiao L, et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
3D porous Ti6Al4V-beta-tricalcium phosphate scaffolds directly fabricated by additive manufacturing.Acta Biomater. 2021 May;126:496-510. doi: 10.1016/j.actbio.2021.03.021. Epub 2021 Mar 13. Acta Biomater. 2021. PMID: 33727193
-
3D printed polycaprolactone/β-tricalcium phosphate/carbon nanotube composite - Physical properties and biocompatibility.Heliyon. 2024 Feb 20;10(5):e26071. doi: 10.1016/j.heliyon.2024.e26071. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38468962 Free PMC article.
-
Beta-tricalcium phosphate enhanced mechanical and biological properties of 3D-printed polyhydroxyalkanoates scaffold for bone tissue engineering.Int J Biol Macromol. 2022 Jun 1;209(Pt A):1553-1561. doi: 10.1016/j.ijbiomac.2022.04.056. Epub 2022 Apr 18. Int J Biol Macromol. 2022. PMID: 35439474
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
-
3D printed porous ceramic scaffolds for bone tissue engineering: a review.Biomater Sci. 2017 Aug 22;5(9):1690-1698. doi: 10.1039/c7bm00315c. Biomater Sci. 2017. PMID: 28686244 Review.
Cited by
-
Prospects and challenges for the application of tissue engineering technologies in the treatment of bone infections.Bone Res. 2024 May 14;12(1):28. doi: 10.1038/s41413-024-00332-w. Bone Res. 2024. PMID: 38744863 Free PMC article. Review.
-
3D nanocomposites of β-TCP-H3BO3-Cu with improved mechanical and biological performances for bone regeneration applications.Sci Rep. 2025 Jan 25;15(1):3224. doi: 10.1038/s41598-025-87988-4. Sci Rep. 2025. PMID: 39863796 Free PMC article.
-
Research progress of 3D printed poly (ether ether ketone) in the reconstruction of craniomaxillofacial bone defects.Front Bioeng Biotechnol. 2023 Aug 16;11:1259696. doi: 10.3389/fbioe.2023.1259696. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37662437 Free PMC article. Review.
-
Enhancing bone regeneration through 3D printed biphasic calcium phosphate scaffolds featuring graded pore sizes.Bioact Mater. 2024 Dec 9;46:21-36. doi: 10.1016/j.bioactmat.2024.11.024. eCollection 2025 Apr. Bioact Mater. 2024. PMID: 39734570 Free PMC article.
-
Enhancing Bone Repair with β-TCP-Based Composite Scaffolds: A Review of Design Strategies and Biological Mechanisms.Orthop Res Rev. 2025 Jul 14;17:313-340. doi: 10.2147/ORR.S525959. eCollection 2025. Orthop Res Rev. 2025. PMID: 40688750 Free PMC article. Review.
References
-
- Ribas RG, Schatkoski VM, Do Amaral Montanheiro TL. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram Int . 2019;45((17)):21051–21061.
-
- Brunello G, Sivolella S, Meneghello R. Powderbased 3D printing for bone tissue engineering. Biotechnol Adv . 2016;34((5)):740–753. - PubMed
-
- Palmer S, Gibbons C, Athanasou N. The pathology of bone allograft. J Bone Joint Surg Br . 1999;81((2)):333–335. - PubMed
-
- Meißner R, Bertol L, Rehman MAU. Bioprinted 3D calcium phosphate scaffolds with gentamicin releasing capability. Ceram Int . 2019;45((6)):7090–7094.
LinkOut - more resources
Full Text Sources
