A review on plasmonic-based heterojunction photocatalysts for degradation of organic pollutants in wastewater
- PMID: 37065680
- PMCID: PMC10039801
- DOI: 10.1007/s10853-023-08391-w
A review on plasmonic-based heterojunction photocatalysts for degradation of organic pollutants in wastewater
Abstract
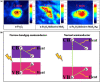
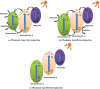
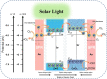
Organic pollutants in wastewater are the biggest problem facing the world today due to population growth, rapid increase in industrialization, urbanization, and technological advancement. There have been numerous attempts to use conventional wastewater treatment techniques to address the issue of worldwide water contamination. However, conventional wastewater treatment has a number of shortcomings, including high operating costs, low efficiency, difficult preparation, fast recombination of charge carriers, generation of secondary waste, and limited light absorption. Therefore, plasmonic-based heterojunction photocatalysts have attracted much attention as a promising method to reduce organic pollutant problems in water due to their excellent efficiency, low operating cost, ease of fabrication, and environmental friendliness. In addition, plasmonic-based heterojunction photocatalysts contain a local surface plasmon resonance that enhances the performance of photocatalysts by improving light absorption and separation of photoexcited charge carriers. This review summarizes the major plasmonic effects in photocatalysts, including hot electron, local field effect, and photothermal effect, and explains the plasmonic-based heterojunction photocatalysts with five junction systems for the degradation of pollutants. Recent work on the development of plasmonic-based heterojunction photocatalysts for the degradation of various organic pollutants in wastewater is also discussed. Lastly, the conclusions and challenges are briefly described and the direction of future development of heterojunction photocatalysts with plasmonic materials is explored. This review could serve as a guide for the understanding, investigation, and construction of plasmonic-based heterojunction photocatalysts for various organic pollutants degradation.
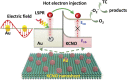
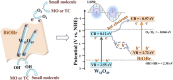
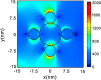
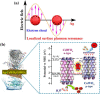
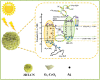
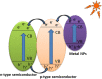
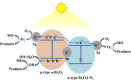
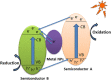
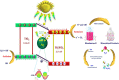
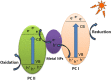
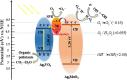
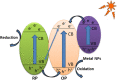
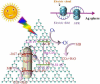
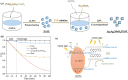
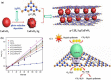
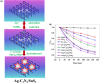
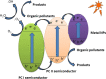
Graphical abstract: Herein, the plasmonic effects in photocatalysts, such as hot electrons, local field effect, and photothermal effect, as well as the plasmonic-based heterojunction photocatalysts with five junction systems for the degradation of pollutants are explained. Recent work on plasmonic-based heterojunction photocatalysts for the degradation of various organic pollutants in wastewater such as dyes, pesticides, phenols, and antibiotics is discussed. Challenges and future developments are also described.
© The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature 2023, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this work.
Figures






Similar articles
-
A critical review on strategies for improving efficiency of BaTiO3-based photocatalysts for wastewater treatment.J Environ Manage. 2021 Jul 15;290:112679. doi: 10.1016/j.jenvman.2021.112679. Epub 2021 Apr 24. J Environ Manage. 2021. PMID: 33901825 Review.
-
Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances.Chem Soc Rev. 2014 Aug 7;43(15):5234-44. doi: 10.1039/c4cs00126e. Chem Soc Rev. 2014. PMID: 24841176
-
A critical review on relationship of CeO2-based photocatalyst towards mechanistic degradation of organic pollutant.Chemosphere. 2022 Jan;286(Pt 1):131651. doi: 10.1016/j.chemosphere.2021.131651. Epub 2021 Jul 24. Chemosphere. 2022. PMID: 34346345 Review.
-
2D/2D Heterojunction systems for the removal of organic pollutants: A review.Adv Colloid Interface Sci. 2021 Nov;297:102540. doi: 10.1016/j.cis.2021.102540. Epub 2021 Oct 5. Adv Colloid Interface Sci. 2021. PMID: 34634576 Review.
-
Interfacial heterojunction construction by introducing Pd into W18O49 nanowires to promote the visible light-driven photocatalytic degradation of environmental organic pollutants.J Colloid Interface Sci. 2021 May 15;590:518-526. doi: 10.1016/j.jcis.2021.01.067. Epub 2021 Feb 1. J Colloid Interface Sci. 2021. PMID: 33578239
Cited by
-
Plasmonic Ag@Cu2O core-shell nanostructures exhibiting near-infrared photothermal effect.RSC Adv. 2023 Oct 27;13(45):31569-31577. doi: 10.1039/d3ra06712b. eCollection 2023 Oct 26. RSC Adv. 2023. PMID: 37901274 Free PMC article.
-
Hydrothermal Synthesis of Heterostructured g-C3N4/Ag-TiO2 Nanocomposites for Enhanced Photocatalytic Degradation of Organic Pollutants.Materials (Basel). 2023 Aug 7;16(15):5497. doi: 10.3390/ma16155497. Materials (Basel). 2023. PMID: 37570204 Free PMC article.
References
-
- Prasad C, Yang X, Liu Q, et al. Recent advances in MXenes supported semiconductors based photocatalysts: properties, synthesis and photocatalytic applications. J Ind Eng Chem. 2020;85:1–33. doi: 10.1016/j.jiec.2019.12.003. - DOI
-
- Lu H, Li Q, Feng W. Application pogress of O3/UV advanced oxidation technology in the treatment of organic pollutants in water. Sustainability. 2022;14:1556. doi: 10.3390/su14031556. - DOI
-
- Chen D, Cheng Y, Zhou N, et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: a review. J Clean Prod. 2020;268:121725. doi: 10.1016/j.jclepro.2020.121725. - DOI
Publication types
LinkOut - more resources
Full Text Sources
