Defective iron homeostasis and hematological abnormalities in Niemann-Pick disease type C1
- PMID: 37065726
- PMCID: PMC10090865
- DOI: 10.12688/wellcomeopenres.17261.2
Defective iron homeostasis and hematological abnormalities in Niemann-Pick disease type C1
Abstract
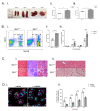
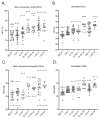
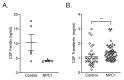
Background: Niemann-Pick disease type C1 (NPC1) is a neurodegenerative lysosomal storage disorder characterized by the accumulation of multiple lipids in the late endosome/lysosomal system and reduced acidic store calcium. The lysosomal system regulates key aspects of iron homeostasis, which prompted us to investigate whether there are hematological abnormalities and iron metabolism defects in NPC1. Methods: Iron-related hematological parameters, systemic and tissue metal ion and relevant hormonal and proteins levels, expression of specific pro-inflammatory mediators and erythrophagocytosis were evaluated in an authentic mouse model and in a large cohort of NPC patients. Results: Significant changes in mean corpuscular volume and corpuscular hemoglobin were detected in Npc1 -/- mice from an early age. Hematocrit, red cell distribution width and hemoglobin changes were observed in late-stage disease animals. Systemic iron deficiency, increased circulating hepcidin, decreased ferritin and abnormal pro-inflammatory cytokine levels were also found. Furthermore, there is evidence of defective erythrophagocytosis in Npc1 -/- mice and in an in vitro NPC1 cellular model. Comparable hematological changes, including low normal serum iron and transferrin saturation and low cerebrospinal fluid ferritin were confirmed in NPC1 patients. Conclusions: These data suggest loss of iron homeostasis and hematological abnormalities in NPC1 may contribute to the pathophysiology of this disease.
Keywords: Niemann-Pick disease type C; haematology; iron; lysosomal storage diseases; lysosome.
Copyright: © 2023 Chen OCW et al.
Conflict of interest statement
Competing interests: F.M.P. is a consultant to and co-founder of Intrabio. Other authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

