Osteocalcin modulates parathyroid cell function in human parathyroid tumors
- PMID: 37065733
- PMCID: PMC10098338
- DOI: 10.3389/fendo.2023.1129930
Osteocalcin modulates parathyroid cell function in human parathyroid tumors
Abstract
Introduction: The bone matrix protein osteocalcin (OC), secreted by osteoblasts, displays endocrine effects. We tested the hypothesis that OC modulates parathyroid tumor cell function.
Methods: Primary cell cultures derived from parathyroid adenomas (PAds) and HEK293 cells transiently transfected with the putative OC receptor GPRC6A or the calcium sensing receptor (CASR) were used as experimental models to investigate γ-carboxylated OC (GlaOC) or uncarboxylated OC (GluOC) modulation of intracellular signaling.
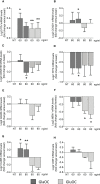
Results: In primary cell cultures derived from PAds, incubation with GlaOC or GluOC modulated intracellular signaling, inhibiting pERK/ERK and increasing active β-catenin levels. GlaOC increased the expression of PTH, CCND1 and CASR, and reduced CDKN1B/p27 and TP73. GluOC stimulated transcription of PTH, and inhibited MEN1 expression. Moreover, GlaOC and GluOC reduced staurosporin-induced caspase 3/7 activity. The putative OC receptor GPRC6A was detected in normal and tumor parathyroids at membrane or cytoplasmic level in cells scattered throughout the parenchyma. In PAds, the membrane expression levels of GPRC6A and its closest homolog CASR positively correlated; GPRC6A protein levels positively correlated with circulating ionized and total calcium, and PTH levels of the patients harboring the analyzed PAds. Using HEK293A transiently transfected with either GPRC6A or CASR, and PAds-derived cells silenced for CASR, we showed that GlaOC and GluOC modulated pERK/ERK and active β-catenin mainly through CASR activation.
Conclusion: Parathyroid gland emerges as a novel target of the bone secreted hormone osteocalcin, which may modulate tumor parathyroid CASR sensitivity and parathyroid cell apoptosis.
Keywords: CASR; ERK; GPRC6A; beta-catenin; osteocalcin; parathyroid tumor.
Copyright © 2023 Verdelli, Tavanti, Forno, Vaira, Maggiore, Vicentini, Dalino Ciaramella, Perticone, Lombardi and Corbetta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

