ZBTB7A as a novel vulnerability in neuroendocrine prostate cancer
- PMID: 37065756
- PMCID: PMC10090553
- DOI: 10.3389/fendo.2023.1093332
ZBTB7A as a novel vulnerability in neuroendocrine prostate cancer
Abstract
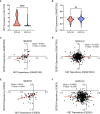
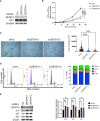
Neuroendocrine prostate cancer (NEPC) is a highly aggressive subtype of prostate cancer. NEPC is characterized by the loss of androgen receptor (AR) signaling and transdifferentiation toward small-cell neuroendocrine (SCN) phenotypes, which results in resistance to AR-targeted therapy. NEPC resembles other SCN carcinomas clinically, histologically and in gene expression. Here, we leveraged SCN phenotype scores of various cancer cell lines and gene depletion screens from the Cancer Dependency Map (DepMap) to identify vulnerabilities in NEPC. We discovered ZBTB7A, a transcription factor, as a candidate promoting the progression of NEPC. Cancer cells with high SCN phenotype scores showed a strong dependency on RET kinase activity with a high correlation between RET and ZBTB7A dependencies in these cells. Utilizing informatic modeling of whole transcriptome sequencing data from patient samples, we identified distinct gene networking patterns of ZBTB7A in NEPC versus prostate adenocarcinoma. Specifically, we observed a robust association of ZBTB7A with genes promoting cell cycle progression, including apoptosis regulating genes. Silencing ZBTB7A in a NEPC cell line confirmed the dependency on ZBTB7A for cell growth via suppression of the G1/S transition in the cell cycle and induction of apoptosis. Collectively, our results highlight the oncogenic function of ZBTB7A in NEPC and emphasize the value of ZBTB7A as a promising therapeutic strategy for targeting NEPC tumors.
Keywords: RET receptor tyrosine kinase; ZBTB7A; cancer dependency map (DepMap); castration-resistant prostate cancer (CRPC); cell cycle; gene network; neuroendocrine prostate cancer (NEPC); small-cell neuroendocrine (SCN).
Copyright © 2023 Bae, Bergom, Day, Greene, Sychev, Larson, Corey, Plymate, Freedman, Hwang and Drake.
Conflict of interest statement
EC is consultant of Dotquant and received research funding via institutional SRAs from AbbVie, Janssen Research, Gilead, Zenith Epigenetics, Bayer, GSK, Astra Zeneca, Kronos, Foghorn, MacroGenics, and Forma Pharmaceuticals. JMD has no conflicts relevant to this work. However, he holds equity in and serves as Chief Scientific Officer of Astrin Biosciences. This interest has been reviewed and managed by the University of Minnesota in accordance with its Conflict of Interest policies. None of these companies contributed to or directed any of the research reported in this article. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
ONECUT2 is a driver of neuroendocrine prostate cancer.Nat Commun. 2019 Jan 17;10(1):278. doi: 10.1038/s41467-018-08133-6. Nat Commun. 2019. PMID: 30655535 Free PMC article.
-
SRRM4 Drives Neuroendocrine Transdifferentiation of Prostate Adenocarcinoma Under Androgen Receptor Pathway Inhibition.Eur Urol. 2017 Jan;71(1):68-78. doi: 10.1016/j.eururo.2016.04.028. Epub 2016 May 11. Eur Urol. 2017. PMID: 27180064
-
Identification of Novel Diagnosis Biomarkers for Therapy-Related Neuroendocrine Prostate Cancer.Pathol Oncol Res. 2021 Sep 27;27:1609968. doi: 10.3389/pore.2021.1609968. eCollection 2021. Pathol Oncol Res. 2021. PMID: 34646089 Free PMC article.
-
Emerging Therapeutic Targets of Neuroendocrine Prostate Cancer.Curr Oncol Rep. 2025 Apr;27(4):362-374. doi: 10.1007/s11912-025-01643-9. Epub 2025 Feb 27. Curr Oncol Rep. 2025. PMID: 40011325 Review.
-
Molecular events in neuroendocrine prostate cancer development.Nat Rev Urol. 2021 Oct;18(10):581-596. doi: 10.1038/s41585-021-00490-0. Epub 2021 Jul 21. Nat Rev Urol. 2021. PMID: 34290447 Free PMC article. Review.
Cited by
-
New Insights into Potential Therapeutic Targets for Neuroendocrine Prostate Cancer: From Bench to Clinic.Research (Wash D C). 2025 Jul 31;8:0791. doi: 10.34133/research.0791. eCollection 2025. Research (Wash D C). 2025. PMID: 40746825 Free PMC article. Review.
-
Therapeutic Exploitation of Neuroendocrine Transdifferentiation Drivers in Prostate Cancer.Cells. 2024 Dec 3;13(23):1999. doi: 10.3390/cells13231999. Cells. 2024. PMID: 39682746 Free PMC article. Review.
References
-
- Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, et al. . Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J Clin Oncol (2018) 36(24):2492–503. doi: 10.1200/JCO.2017.77.6880 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

