Mechanisms involved in cancer stem cell resistance in head and neck squamous cell carcinoma
- PMID: 37065869
- PMCID: PMC10099599
- DOI: 10.20517/cdr.2022.107
Mechanisms involved in cancer stem cell resistance in head and neck squamous cell carcinoma
Abstract
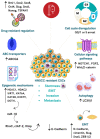
Despite scientific advances in the Oncology field, cancer remains a leading cause of death worldwide. Molecular and cellular heterogeneity of head and neck squamous cell carcinoma (HNSCC) is a significant contributor to the unpredictability of the clinical response and failure in cancer treatment. Cancer stem cells (CSCs) are recognized as a subpopulation of tumor cells that can drive and maintain tumorigenesis and metastasis, leading to poor prognosis in different types of cancer. CSCs exhibit a high level of plasticity, quickly adapting to the tumor microenvironment changes, and are intrinsically resistant to current chemo and radiotherapies. The mechanisms of CSC-mediated therapy resistance are not fully understood. However, they include different strategies used by CSCs to overcome challenges imposed by treatment, such as activation of DNA repair system, anti-apoptotic mechanisms, acquisition of quiescent state and Epithelial-mesenchymal transition, increased drug efflux capacity, hypoxic environment, protection by the CSC niche, overexpression of stemness related genes, and immune surveillance. Complete elimination of CSCs seems to be the main target for achieving tumor control and improving overall survival for cancer patients. This review will focus on the multi-factorial mechanisms by which CSCs are resistant to radiotherapy and chemotherapy in HNSCC, supporting the use of possible strategies to overcome therapy failure.
Keywords: Head and neck squamous cell carcinoma; cancer stem cell; chemotherapy; radiotherapy; therapy resistance.
© The Author(s) 2023.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures

References
-
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
