Prevalence and Factors Associated with Drooling in Parkinson's Disease: Results from a Longitudinal Prospective Cohort and Comparison with a Control Group
- PMID: 37065970
- PMCID: PMC10101739
- DOI: 10.1155/2023/3104425
Prevalence and Factors Associated with Drooling in Parkinson's Disease: Results from a Longitudinal Prospective Cohort and Comparison with a Control Group
Abstract
Introduction: Drooling in Parkinson's disease (PD) is frequent but often goes underrecognized. Our aim was to examine the prevalence of drooling in a PD cohort and compare it with a control group. Specifically, we identified factors associated with drooling and conducted subanalyses in a subgroup of very early PD patients. Patients and Methods. PD patients who were recruited from January 2016 to November 2017 (baseline visit; V0) and evaluated again at a 2-year ± 30-day follow-up (V2) from 35 centers in Spain from the COPPADIS cohort were included in this longitudinal prospective study. Subjects were classified as with or without drooling according to item 19 of the NMSS (Nonmotor Symptoms Scale) at V0, V1 (1-year ± 15 days), and V2 for patients and at V0 and V2 for controls.
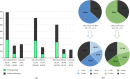
Results: The frequency of drooling in PD patients was 40.1% (277/691) at V0 (2.4% (5/201) in controls; p < 0.0001), 43.7% (264/604) at V1, and 48.2% (242/502) at V2 (3.2% (4/124) in controls; p < 0.0001), with a period prevalence of 63.6% (306/481). Being older (OR = 1.032; p = 0.012), being male (OR = 2.333; p < 0.0001), having greater nonmotor symptom (NMS) burden at the baseline (NMSS total score at V0; OR = 1.020; p < 0.0001), and having a greater increase in the NMS burden from V0 to V2 (change in the NMSS total score from V0 to V2; OR = 1.012; p < 0.0001) were identified as independent predictors of drooling after the 2-year follow-up. Similar results were observed in the group of patients with ≤2 years since symptom onset, with a cumulative prevalence of 64.6% and a higher score on the UPDRS-III at V0 (OR = 1.121; p = 0.007) as a predictor of drooling at V2.
Conclusion: Drooling is frequent in PD patients even at the initial onset of the disease and is associated with a greater motor severity and NMS burden.
Copyright © 2023 Diego Santos-García et al.
Conflict of interest statement
Santos García D. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, Teva, Archímedes, Esteve, Stada, and grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017–2020 por el proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”). De Deus Fonticoba T.: None. Cores Bartolomé C. has received honoraria for educational presentations and advice service by Lundbeck and UCB Pharma. Feal Painceiras M. J.: None. Íñiguez Alvarado MC: None. Jesús S. has received honoraria from AbbVie, Bial, Merz, UCB, and Zambon and holds the competitive contract “Juan Rodés” supported by the Instituto de Salud Carlos III. She has received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459-2018). Buongiorno M. T.: Planellas LL.: None. Cosgaya M.: None. García Caldentey J. has received honoraria for educational presentations and advice service by Qualigen, Nutricia, Abbvie, Italfarmaco, UCB Pharma, Lundbeck, Zambon, Bial, and Teva. Caballol N. has received honoraria from Bial, Italfarmaco, Qualigen, Zambon, UCB, Teva, and KRKA and sponsorship from Zambon, TEVA, and Abbvie for attending medical conferences. Legarda I. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva. Hernández Vara J. has received travel bursaries and educational grants from Abbvie and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco, and Sanofi-Genzyme. Cabo I. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial. López Manzanares L.: Compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon. González Aramburu I.: None. Ávila Rivera MA. has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva and sponsorship from Zambon and Teva for attending conferences. Gómez Mayordomo V.: None. Nogueira V.: None. Puente V. has served as consultant for Abbvie and Zambon; has received grant/research from Abbvie. Dotor García-Soto J.: Compensated advisory services, consulting, research grant support, or speaker honoraria: Merck, Sanofi-Genzyme, Allergan, Biogen, Roche, UCB, and Novartis. Borrué C.: None. Solano Vila B. has received honoraria for educational presentations and advice service by UCB, Zambon, Teva, Abbvie, and Bial. Álvarez Sauco M. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva. Vela L. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. Escalante S. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial. Cubo E.: Travel grants: Abbvie, Allergan, Boston; Lecturing honoraria: Abbvie, International Parkinson´s disease Movement Disorder Society. Carrillo Padilla F. has received honoraria from Zambon (SEN Congress assistance). Martínez Castrillo JC. has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie. He has received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz. Sánchez Alonso P. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. Alonso Losada M. G. has received honoraria for educational presentations and advice service by Zambon and Bial. López Ariztegui N. has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial. Gastón I. has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts, and Zambon. Kulisevsky J.: (1) Consulting fees: Roche, Zambon; (2) Stock/allotment: No; (3) Patent royalties/licensing fees: No; (4) Honoraria (e.g. lecture fees): Zambon, Teva, Bial, UCB; (5) Fees for promotional materials: No; (6) Research funding: Roche, Zambon, Ciberned; Instituto de SaludCarlos III; FundacióLa Maratóde TV3; (7) Scholarship from corporation: No; (8) Corporate laboratory funding: No; (9) Others (e.g. trips, travel, or gifts): No. Blázquez Estrada M. has received honoraria for educational presentations and advice service by Abbvie, Abbott, UCB Pharma, Allergan, Zambon, Bial, and Qualigen. Seijo M. has received honoraria for educational services from KRKA, UCB, Zambon, Bial; travel grants from Daiichi and Roche. Ruiz Martínez J. has received honoraria for educational presentations, attending medical conferences, and advice service by Abbvie, UCB Pharma, Zambon, Italfarmaco, Bial, and Teva. Valero C. has received honoraria for educational services from Zambon, Abbvie, and UCB. Kurtis M. has received honoraria from Bial, the Spanish Neurology Society, and the International and Movement Disorders Society. de Fábregues O. has received honoraria for educational presentations and advice service by Bial, Zambon, Abbvie, KRKA, and Teva. González Ardura J. has recieved honoraria for speking from italofarma, Krka, Genzyme, UCB, Esteve, Psyma iberica marketing research SL and Ferrer, course grant from Teva and travel grant from Merck. Alonso Redondo R.: None. Ordás C.: None. López Díaz L. M. has received honoraria from UCB, Lundbeck, and KRKA. McAfee D.: None. Martínez-Martin P. has received honoraria from National School of Public Health (ISCIII), Editori-al Viguera and Takeda Pharmaceuticals for lecturing in courses, and from the International Parkinson and Movement Disorder Society (MDS) for management of the Program on Rating Scales. Mir P. has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon and have received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación) and by Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [PI-0437-2012, PI-0471-2013], the Sociedad Andaluza de Neurología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, the Fundación Mutua Madrileña.
Figures

Similar articles
-
Diplopia Is Frequent and Associated with Motor and Non-Motor Severity in Parkinson's Disease: Results from the COPPADIS Cohort at 2-Year Follow-Up.Diagnostics (Basel). 2021 Dec 17;11(12):2380. doi: 10.3390/diagnostics11122380. Diagnostics (Basel). 2021. PMID: 34943619 Free PMC article.
-
Predictors of Global Non-Motor Symptoms Burden Progression in Parkinson's Disease. Results from the COPPADIS Cohort at 2-Year Follow-Up.J Pers Med. 2021 Jun 30;11(7):626. doi: 10.3390/jpm11070626. J Pers Med. 2021. PMID: 34209166 Free PMC article.
-
Motor Fluctuations Development Is Associated with Non-Motor Symptoms Burden Progression in Parkinson's Disease Patients: A 2-Year Follow-Up Study.Diagnostics (Basel). 2022 May 5;12(5):1147. doi: 10.3390/diagnostics12051147. Diagnostics (Basel). 2022. PMID: 35626303 Free PMC article.
-
Pathophysiology and Symptomatology of Drooling in Parkinson's Disease.Healthcare (Basel). 2022 Mar 11;10(3):516. doi: 10.3390/healthcare10030516. Healthcare (Basel). 2022. PMID: 35326994 Free PMC article. Review.
-
Drooling rating scales in Parkinson's disease: A systematic review.Parkinsonism Relat Disord. 2021 Oct;91:173-180. doi: 10.1016/j.parkreldis.2021.09.012. Epub 2021 Sep 21. Parkinsonism Relat Disord. 2021. PMID: 34583888
Cited by
-
Predictors of drooling severity in people with Parkinson's disease.J Neurol. 2025 Jan 15;272(2):129. doi: 10.1007/s00415-024-12739-w. J Neurol. 2025. PMID: 39812680
-
Recommendations for a paradigm shift in approach to increase the recognition and treatment of sialorrhea in Parkinson's disease.Clin Park Relat Disord. 2023 Oct 11;9:100223. doi: 10.1016/j.prdoa.2023.100223. eCollection 2023. Clin Park Relat Disord. 2023. PMID: 38021341 Free PMC article. Review.
References
-
- Miller N., Walshe M., Walker R. W. Research and Reviews in Parkinsonism . 2019;9:17–28. doi: 10.2147/jprls.s177409. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

