Theoretical considerations and supporting evidence for the primary role of source geometry on field potential amplitude and spatial extent
- PMID: 37066073
- PMCID: PMC10097999
- DOI: 10.3389/fncel.2023.1129097
Theoretical considerations and supporting evidence for the primary role of source geometry on field potential amplitude and spatial extent
Abstract
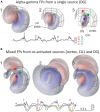
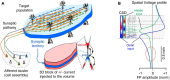
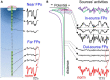
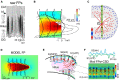
Field potential (FP) recording is an accessible means to capture the shifts in the activity of neuron populations. However, the spatial and composite nature of these signals has largely been ignored, at least until it became technically possible to separate activities from co-activated sources in different structures or those that overlap in a volume. The pathway-specificity of mesoscopic sources has provided an anatomical reference that facilitates transcending from theoretical analysis to the exploration of real brain structures. We review computational and experimental findings that indicate how prioritizing the spatial geometry and density of sources, as opposed to the distance to the recording site, better defines the amplitudes and spatial reach of FPs. The role of geometry is enhanced by considering that zones of the active populations that act as sources or sinks of current may arrange differently with respect to each other, and have different geometry and densities. Thus, observations that seem counterintuitive in the scheme of distance-based logic alone can now be explained. For example, geometric factors explain why some structures produce FPs and others do not, why different FP motifs generated in the same structure extend far while others remain local, why factors like the size of an active population or the strong synchronicity of its neurons may fail to affect FPs, or why the rate of FP decay varies in different directions. These considerations are exemplified in large structures like the cortex and hippocampus, in which the role of geometrical elements and regional activation in shaping well-known FP oscillations generally go unnoticed. Discovering the geometry of the sources in play will decrease the risk of population or pathway misassignments based solely on the FP amplitude or temporal pattern.
Keywords: LFP; current source; field potential; network oscillations; source geometry; source localization; spatial reach; volume conduction.
Copyright © 2023 Herreras, Torres, Makarov and Makarova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Site-dependent shaping of field potential waveforms.Cereb Cortex. 2023 Mar 21;33(7):3636-3650. doi: 10.1093/cercor/bhac297. Cereb Cortex. 2023. PMID: 35972425 Free PMC article.
-
Cross Laminar Traveling Components of Field Potentials due to Volume Conduction of Non-Traveling Neuronal Activity in Macaque Sensory Cortices.J Neurosci. 2021 Sep 8;41(36):7578-7590. doi: 10.1523/JNEUROSCI.3225-20.2021. Epub 2021 Jul 28. J Neurosci. 2021. PMID: 34321312 Free PMC article.
-
Primary Generators of Visually Evoked Field Potentials Recorded in the Macaque Auditory Cortex.J Neurosci. 2017 Oct 18;37(42):10139-10153. doi: 10.1523/JNEUROSCI.3800-16.2017. Epub 2017 Sep 18. J Neurosci. 2017. PMID: 28924008 Free PMC article.
-
Local Field Potentials: Myths and Misunderstandings.Front Neural Circuits. 2016 Dec 15;10:101. doi: 10.3389/fncir.2016.00101. eCollection 2016. Front Neural Circuits. 2016. PMID: 28018180 Free PMC article. Review.
-
New uses of LFPs: Pathway-specific threads obtained through spatial discrimination.Neuroscience. 2015 Dec 3;310:486-503. doi: 10.1016/j.neuroscience.2015.09.054. Epub 2015 Sep 28. Neuroscience. 2015. PMID: 26415769 Review.
Cited by
-
Uncorrelated bilateral cortical input becomes timed across hippocampal subfields for long waves whereas gamma waves are largely ipsilateral.Front Cell Neurosci. 2023 Jul 27;17:1217081. doi: 10.3389/fncel.2023.1217081. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37576568 Free PMC article.
-
Diversity of cortical activity changes beyond depression during Spreading Depolarizations.Nat Commun. 2023 Nov 25;14(1):7729. doi: 10.1038/s41467-023-43509-3. Nat Commun. 2023. PMID: 38007508 Free PMC article.
-
Brain sources composing irregular field potentials have unique temporal signatures.Cereb Cortex. 2025 Jun 4;35(6):bhaf135. doi: 10.1093/cercor/bhaf135. Cereb Cortex. 2025. PMID: 40511993 Free PMC article.
-
Intracranial Voltage Profiles from Untangled Human Deep Sources Reveal Multisource Composition and Source Allocation Bias.J Neurosci. 2025 Jan 1;45(1):e0695242024. doi: 10.1523/JNEUROSCI.0695-24.2024. J Neurosci. 2025. PMID: 39481886 Free PMC article.
References
-
- Abeles M. (1991). Corticonics: neural circuits of the cerebral cortex. Cambridge: Cambridge University Press.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

