This is a preprint.
Control of working memory maintenance by theta-gamma phase amplitude coupling of human hippocampal neurons
- PMID: 37066145
- PMCID: PMC10104113
- DOI: 10.1101/2023.04.05.535772
Control of working memory maintenance by theta-gamma phase amplitude coupling of human hippocampal neurons
Update in
-
Control of working memory by phase-amplitude coupling of human hippocampal neurons.Nature. 2024 May;629(8011):393-401. doi: 10.1038/s41586-024-07309-z. Epub 2024 Apr 17. Nature. 2024. PMID: 38632400 Free PMC article.
Abstract
Retaining information in working memory (WM) is a demanding process that relies on cognitive control to protect memoranda-specific persistent activity from interference. How cognitive control regulates WM storage, however, remains unknown. We hypothesized that interactions of frontal control and hippocampal persistent activity are coordinated by theta-gamma phase amplitude coupling (TG-PAC). We recorded single neurons in the human medial temporal and frontal lobe while patients maintained multiple items in WM. In the hippocampus, TG-PAC was indicative of WM load and quality. We identified cells that selectively spiked during nonlinear interactions of theta phase and gamma amplitude. These PAC neurons were more strongly coordinated with frontal theta activity when cognitive control demand was high, and they introduced information-enhancing and behaviorally relevant noise correlations with persistently active neurons in the hippocampus. We show that TG-PAC integrates cognitive control and WM storage to improve the fidelity of WM representations and facilitate behavior.
Conflict of interest statement
Competing Interests Statement Authors declare no competing interests.
Figures
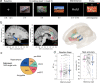
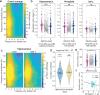




References
-
- Al-Daffaie K, Khan S (2017) Logistic regression for circular data. AIP Conference Proceedings 1842:030022. 10.1063/1.4982860 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
