This is a preprint.
Stepwise design of pseudosymmetric protein hetero-oligomers
- PMID: 37066191
- PMCID: PMC10104133
- DOI: 10.1101/2023.04.07.535760
Stepwise design of pseudosymmetric protein hetero-oligomers
Update in
-
Design of pseudosymmetric protein hetero-oligomers.Nat Commun. 2024 Dec 18;15(1):10684. doi: 10.1038/s41467-024-54913-8. Nat Commun. 2024. PMID: 39695145 Free PMC article.
Abstract
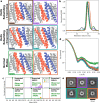
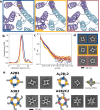
Pseudosymmetric hetero-oligomers with three or more unique subunits with overall structural (but not sequence) symmetry play key roles in biology, and systematic approaches for generating such proteins de novo would provide new routes to controlling cell signaling and designing complex protein materials. However, the de novo design of protein hetero-oligomers with three or more distinct chains with nearly identical structures is a challenging problem because it requires the accurate design of multiple protein-protein interfaces simultaneously. Here, we describe a divide-and-conquer approach that breaks the multiple-interface design challenge into a set of more tractable symmetric single-interface redesign problems, followed by structural recombination of the validated homo-oligomers into pseudosymmetric hetero-oligomers. Starting from de novo designed circular homo-oligomers composed of 9 or 24 tandemly repeated units, we redesigned the inter-subunit interfaces to generate 15 new homo-oligomers and recombined them to make 17 new hetero-oligomers, including ABC heterotrimers, A2B2 heterotetramers, and A3B3 and A2B2C2 heterohexamers which assemble with high structural specificity. The symmetric homo-oligomers and pseudosymmetric hetero-oligomers generated for each system share a common backbone, and hence are ideal building blocks for generating and functionalizing larger symmetric assemblies.
Conflict of interest statement
Competing interests A provisional patent application has been filed (63/486,872) by the University of Washington, listing R.D.K., Nicholas Woodall, B.I.M.W., B.L.S., S.L., and D.B. as inventors or contributors.
Figures



References
-
- Lee S. Design of T=4 de novo protein cages using pseudosymmetric hetero-oligomers. Prep.
-
- Levy E. D. & Teichmann S. A. Chapter Two - Structural, Evolutionary, and Assembly Principles of Protein Oligomerization. in Oligomerization in Health and Disease (eds. Giraldo J. & Ciruela F.) vol. 117 25–51 (Academic Press, 2013). - PubMed
-
- Lang D., Thoma R., Henn-Sax M., Sterner R. & Wilmanns M. Structural Evidence for Evolution of the β/α Barrel Scaffold by Gene Duplication and Fusion. Science 289, 1546–1550 (2000). - PubMed
-
- Khusial P., Plaag R. & Zieve G. W. LSm proteins form heptameric rings that bind to RNA via repeating motifs. Trends Biochem. Sci. 30, 522–528 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
