This is a preprint.
sHIV-1 drug resistance in people on dolutegravir-based ART: Collaborative analysis of cohort studies
- PMID: 37066200
- PMCID: PMC10104228
- DOI: 10.1101/2023.04.05.23288183
sHIV-1 drug resistance in people on dolutegravir-based ART: Collaborative analysis of cohort studies
Update in
-
HIV-1 drug resistance in people on dolutegravir-based antiretroviral therapy: a collaborative cohort analysis.Lancet HIV. 2023 Nov;10(11):e733-e741. doi: 10.1016/S2352-3018(23)00228-X. Epub 2023 Oct 10. Lancet HIV. 2023. PMID: 37832567 Free PMC article.
Abstract
Background: The widespread use of the integrase strand transfer inhibitor (INSTI) dolutegravir (DTG) in first- and second-line antiretroviral therapy (ART) may facilitate emerging resistance. We combined data from HIV cohorts to examine patterns of drug resistance mutations (DRMs) and identify risk factors for DTG resistance.
Methods: Eight cohorts from Canada, Europe, and South Africa contributed data on individuals with genotypic resistance testing on DTG-based ART. Resistance levels were categorised using the Stanford algorithm. We identified risk factors for resistance using mixed-effects ordinal logistic regression models.
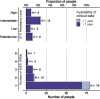
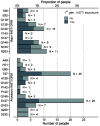
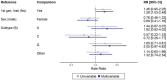
Results: We included 750 people with genotypic resistance testing on DTG-based ART between 2013 and 2022. Most had HIV subtype B (N=444, 59·2%) and were treatment-experienced; 134 (17.9%) were on DTG dual and 19 (2.5%) on DTG monotherapy. INSTI DRMs were detected in 100 (13·3%) individuals; 21 (2·8%) had more than one mutation. Most (N=713, 95·1%) were susceptible to DTG, 8 (1·1%) had potential-low, 5 (0·7%) low, 18 (2·4%) intermediate and 6 (0·8%) high-level DTG resistance. The risk of DTG resistance was higher on DTG monotherapy (adjusted odds ratio (aOR) 37·25, 95% CI 11·17 to 124·2) and DTG lamivudine dual therapy (aOR 6·59, 95% CI 1·70 to 25·55) compared to combination ART, and higher in the presence of potential-low/low (aOR 4.62, 95% CI 1.24 to 17.2) or intermediate/high-level (aOR 7·01, 95% CI 2·52 to 19·48) nucleoside reverse transcriptase inhibitors (NRTI) resistance. Viral load on DTG showed a trend towards increased DTG resistance (aOR 1·42, 95% CI 0·92 to 2·19 per standard deviation of log10 area under the viral load curve).
Interpretation: Among people experiencing virological failure on DTG-based ART, INSTI DRMs were uncommon, and DTG resistance was rare. DTG monotherapy and NRTI resistance substantially increased the risk for DTG resistance, which is of concern, notably in resource-limited settings.
Conflict of interest statement
SMI reports grant funding from NIH NIAAA for the work of ART-CC (payment to institution). AvS reports funding from the Dutch Ministry of Health, Welfare and Sport for the maintenance of the ATHENA database, and grant funding from the European Centre for Disease Prevention and Control (ECDC) (payment to institution). MJG reports honoraria as Ad Hoc member of HIV National Advisory Board from Merck, Gilead Sciences, and ViiV, and a leadership position as Medical Director S Alberta HIV clinic. CAS has received funding from Gilead Sciences, ViiV Healthcare and Janssen-Cilag for membership of Data Safety and Monitoring Committees, Advisory Committees and for preparation of educational material. HFG has received personal fees from Merck, Gilead Sciences, ViiV, GSK, Janssen, Johnson and Johnson and Novartis, as an advisor/consultant or for DSMB membership and has received a travel grant from Gilead. JACS reports funding for research in this publication from NIH NIAAA (payment to institution), UK NIHR (payment to institution), and the University of Bern (payment to institution). RL reports support for research in this publication by the National Institute of Allergy & Infectious Diseases of the National Institutes of Health under award number R01AI152772, and support from the National Institute of Allergy & Infectious Diseases of the National Institutes of Health under award number R01AI167699 for a separate project pertaining to HIV treatment strategies. ME reports funding for research in this publication from the Swiss National Science Foundation (32FP30-18949) and the National Institutes of Health (Cooperative Agreement AI069924 and R01 AI152772-01). RK reports funding for research in this publication from the Swiss National Science Foundation and the National Institute of Allergy & Infectious Diseases of the National Institutes of Health, and reports grant funding from Gilead Sciences. All other authors declare no competing interest.
Figures

References
-
- WHO. Update of recommendations on first- and second-line antiretroviral regimens. Geneva, Switzerland:World Health Organization; WHO. 2019. p. 3.
-
- The Lancet HIV. End resistance to dolutegravir roll-out. Lancet HIV. 2020. Sep 1;7(9):e593. - PubMed
-
- Llibre JM, Pulido F, García F, García Deltoro M, Blanco JL, Delgado R. Genetic barrier to resistance for dolutegravir. AIDS Rev. 2015;17(1):56–64. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
