This is a preprint.
Bruton's tyrosine kinase inhibition for the prevention of anaphylaxis: an open-label, phase 2 trial
- PMID: 37066249
- PMCID: PMC10104202
- DOI: 10.21203/rs.3.rs-2757218/v1
Bruton's tyrosine kinase inhibition for the prevention of anaphylaxis: an open-label, phase 2 trial
Update in
-
A phase II study of Bruton's tyrosine kinase inhibition for the prevention of anaphylaxis.J Clin Invest. 2023 Aug 15;133(16):e172335. doi: 10.1172/JCI172335. J Clin Invest. 2023. PMID: 37384412 Free PMC article. Clinical Trial.
Abstract
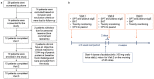
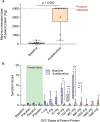
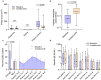
IgE-mediated anaphylaxis is a potentially fatal systemic allergic reaction for which there are no known preventative therapies. Bruton's tyrosine kinase (BTK) is an essential enzyme for IgE-mediated signaling pathways, and is an ideal pharmacologic target to prevent allergic reactions. In this open-label trial (NCT05038904), we evaluated the safety and efficacy of acalabrutinib, a BTK inhibitor that is FDA-approved to treat some B cell malignancies, in preventing clinical reactivity to peanut in adults with IgE-mediated peanut allergy. After undergoing a graded oral peanut challenge to establish their baseline level of clinical reactivity, all patients then received four standard doses of 100 mg acalabrutinib twice daily and underwent repeat food challenge. The primary endpoint was the change in patients' threshold dose of peanut protein to elicit an objective clinical reaction. At baseline, patients tolerated a median of 29 mg of peanut protein before objective clinical reaction. During subsequent food challenge on acalabrutinib, patients' median tolerated dose significantly increased to 4,044 mg (range, 444 - 4,044 mg). 7 of 10 patients tolerated the maximum protocol amount (4,044 mg) of peanut protein with no objective clinical reaction, and the other 3 patients' peanut tolerance increased between 32- and 217-fold. Three patients experienced a total of 4 adverse events that were considered by the investigators to be possibly related to acalabrutinib; all events were transient and nonserious. These results demonstrate that acalabrutinib pretreatment can achieve clinically-relevant increases in patients' tolerance to their food allergen, thereby supporting the need for larger, placebo-controlled trials.
Figures
References
-
- Shaker M.S., et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 145, 1082–1123 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

